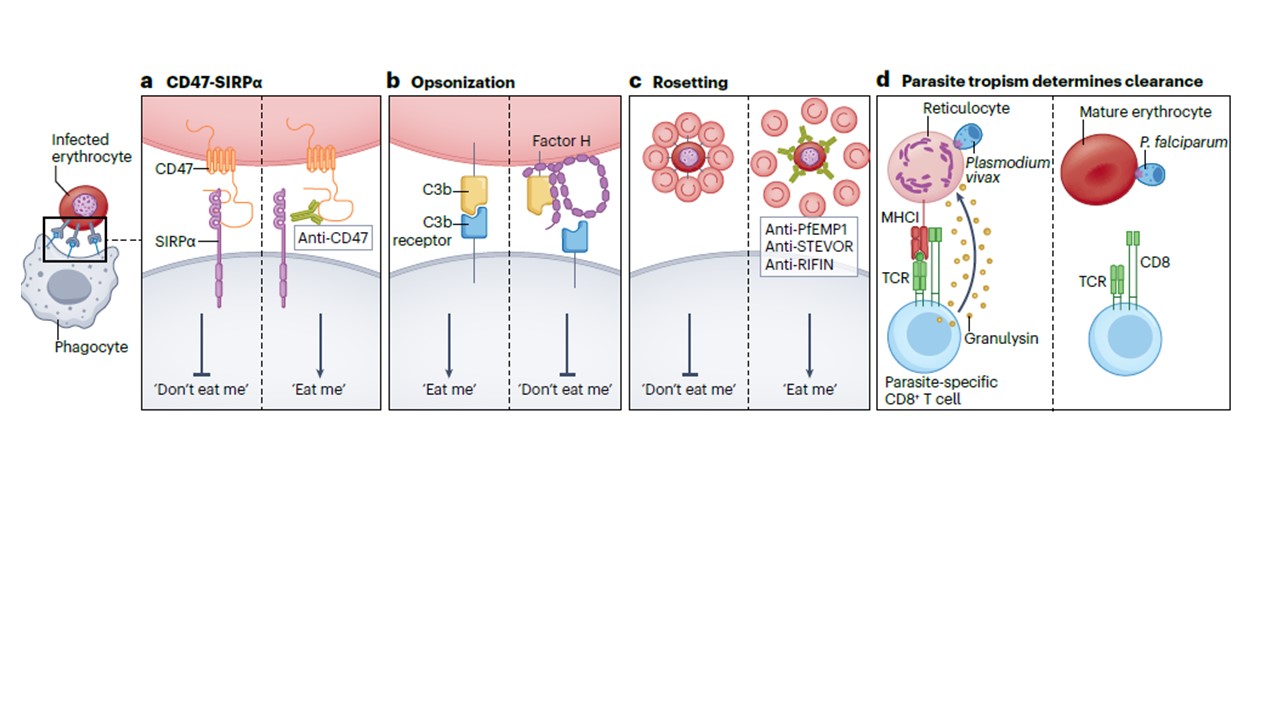
Defective and infected erythrocytes are mainly cleared by macrophages and other phagocytes in the spleen, and accordingly, Plasmodium parasites have developed several mechanisms that delay splenic clearance and promote maintenance of the blood-stage infection. a, Plasmodium infection causes erythrocytes to express CD47, which is recognized by SIRPα on phagocytes as a ‘don’t eat me’ signal. b, Infected erythrocytes recruit the
factor H family of complement inhibitor proteins, which undergo trypsin-resistant binding to the strong opsonin complement factor 3b, thereby preventing
opsonization-induced complement-mediated clearance. c, Infected erythrocytes bear on their surface and secrete parasite-derived proteins that facilitate their adherence to uninfected erythrocytes, thereby forming rosettes that act as a physical barrier to antibody-mediated clearance. d, Finally, Plasmodium parasites preferentially infect mature erythrocytes, which lack major histocompatibility complex (MHC) class I expression and, therefore cannot present parasite antigens to T cells, a trait that enables infected erythrocytes to evade CD8+ T cell-mediated cytotoxicity. The development of cerebral malaria is influenced by the ability of the patient to clear the parasite, mount an effective immune response and modulate endothelial cell responses. All these mechanisms could be potentially used therapeutically to prevent cerebral malaria. PfEMP1, P. falciparum erythrocyte membrane protein 1; SIRPα, signal regulatory protein α; TCR, T cell receptor.
Hadjilaou A, Brandi J, Riehn M, Friese MA, Jacobs T. Pathogenetic mechanisms and treatment targets in cerebral malaria. Nat Rev Neurol. 2023. PMID: 37857843.