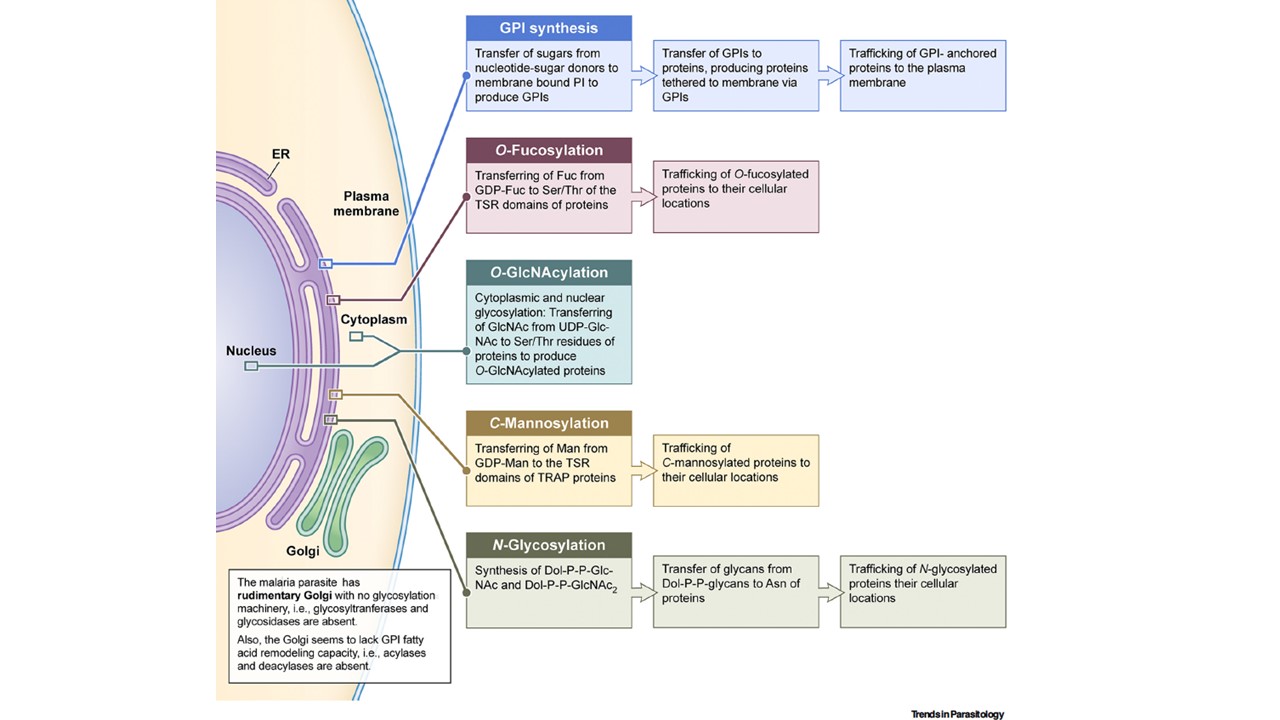
The glycosylation machinery of malaria parasites. Shown here are the nucleus, endoplasmic reticulum (ER), and Golgi of parasites; it is intended to represent the morphologies of all life cycle stages. In malaria parasites, glycosylation is exclusively confined to the ER. Glycosylphosphatidylinositol (GPI) synthesis is initiated in the cytosolic side of the ER, and after two initial steps, the intermediate flips over to the luminal side to complete the production of matured GPIs. GPI anchoring of proteins by the action of transamidase complex also occurs in the ER lumen, and then the GPI-anchored proteins are trafficked to the plasma membrane. N-glycosylation is also initiated in the cytoplasmic side of the ER, producing Dol-P-P-GlcNAc and Dol-P-P-GlcNAc2, which flip over to the lumen of the ER, where the glycan moieties are transferred to proteins by oligosaccharyltransferase (OST). Also, O-fucosylation and tryptophan C-mannosylation occur in the ER lumen (Figure 2E,F). Parasites have a rudimentary Golgi apparatus, and mammalian analogs of glycan-processing enzymes of the Golgi are completely absent in parasites. As such, the glycan moieties of proteins that are added in the ER are not further modified in the Golgi. Malaria parasites also appear to lack fatty acid acylases and deacylases and this could be the reason for the absence GPI fatty acid remodeling in the Golgi.
Gowda DC, Miller LH. Glycosylation in malaria parasites: what do we know? Trends Parasitol. 2024 S1471-4922(23)00311-2. PMID: 38262838.