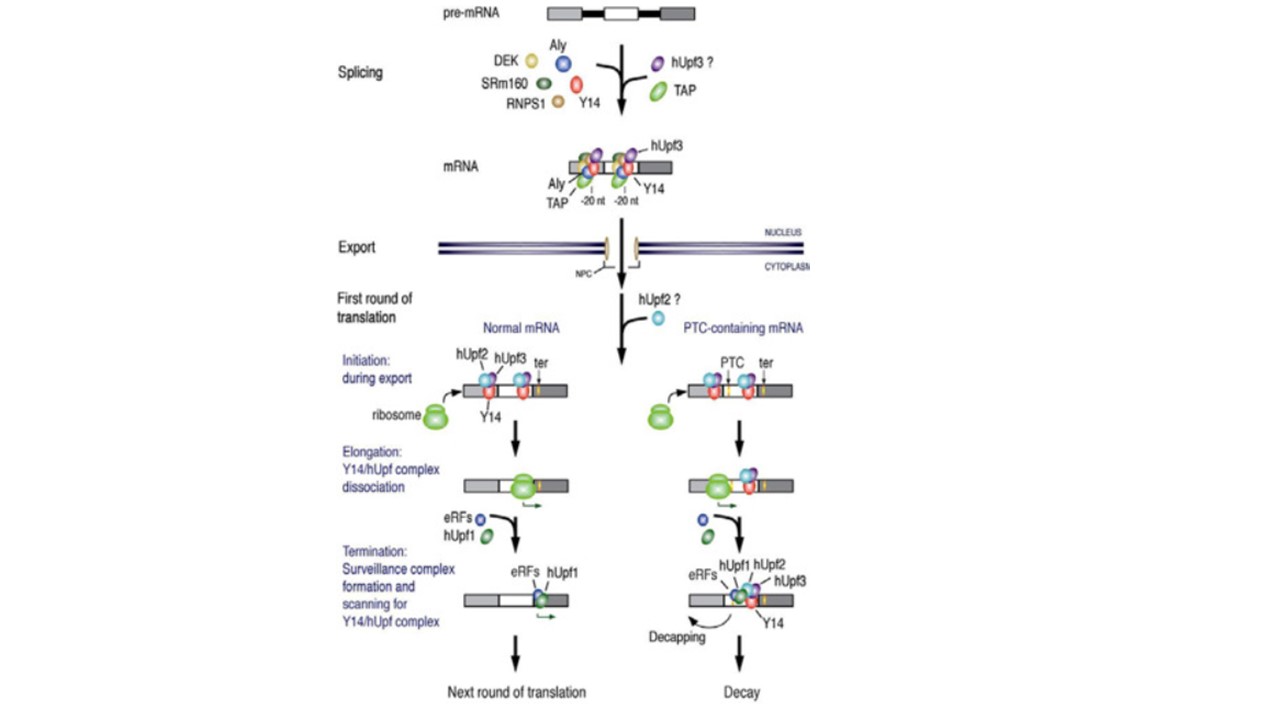
A model for the functional coupling of pre-mRNA splicing and nonsense-mediated decay. The exon-exon junction com-plex is deposited as a result of splicing. hUpf3 becomes associated with the complex while in the nucleus. TAP also binds to the complex and mediates the export. During export, the composition of the complex is changed such that Y14, and possibly hUpf3, remain on the same position while others dissociate. hUpf2 is likely to associate with hUpf3 upon export. The first round of translation is thought to begin while mRNAs are still assoicated with the nuclear pore although in this figure it is depicted for simplicity as if ribosomes bind to the fully exported mRNAs. In normal mRNAs, termination codons (ter) are usually located downstream of the boundary that is ca. -55 nt of the last exon-exon juntion. Thus translating ribosomes remove all the Y14/hUpf2/3 complexes (Y14/hUpf complex) by the time of termination. During termination, hUpf1 and the release factors (eRFs) are recruited and form the surveillance complex which scans for the remaining Y14/hUpf complex. It is not known however when hUpf1, hUpf2, and hUpf3 bind to mRNA. Unless the surveillance complex encounters Y14/hUpf complex, translation goes on to the next round. In the case of mRNA containing a premature termination codon (PTC) upstream of -55 nt of the last exon-exon junction, translation termination occurs before all the Y14/hUpf complex are removed. The remaining Y14/hUpf complex interacts with the surveillance complex and triggers decapping of the mRNA. Kim VN, Dreyfuss G. Nuclear mRNA binding proteins couple pre-mRNA splicing and post-splicing events. Mol Cells. 2001 Aug 31;12(1):1-10. PMID: 11561715.