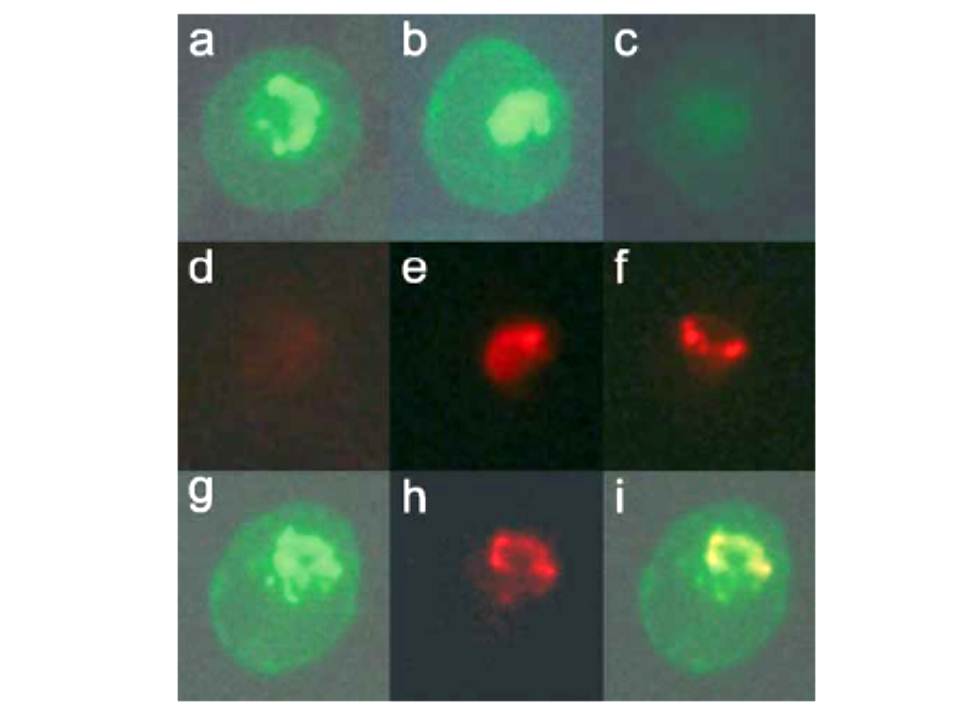
Dual-labeling experiment with anti-COPII components and mAb-134. P. falciparum-infected erythrocytes were fixed with glutaraldehyde and analyzed by immunofluorescence. Dual-labeling experiments were carried out with rabbit antisera against PfSec31p (panels a–f) or PfSar1p (panels g–i) and mAb-134. The samples were then incubated with a mixture of fluorescein-conjugated anti-rabbit IgG and rhodamine-conjugated anti-mouse IgG and examined under filters specific for fluorescein (panels a–c, g) or rhodamine (panels d–f, h). Shown are results of incubating with anti-PfSec31p alone (panels a and d), mAb-134 alone (panels c and f), or a mixture of both antibodies (panels b and e). No rhodamine signal is observed in the sample incubated with anti-PfSec31 (panel d) and no fluorescein signal is observed in the sample incubated with mAb-134 (panel c). Both antibodies predominantly recognize a compartment that is found on one side of the parasite and exhibit substantial overlap, suggesting that this subcellular compartment has some similarities to the endoplasmic reticulum or that this compartment represents a domain of the endoplasmic reticulum.. Samples were incubated with a mixture of rabbit anti-PfSar1p and mAb-134 followed by fluorescein and rhodamine-conjugated secondary antibodies. Shown are the results under filters specific for fluorescein (panel g) or rhodamine (panel h) or a merger between these two images (panel i).
Cortes GT, Winograd E, Wiser MF. Characterization of proteins localized to a subcellular compartment associated with an alternate secretory pathway of the malaria parasite. Mol Biochem Parasitol. 2003 129:127-35. Copyright Elsevier
Other associated proteins
| PFID | Formal Annotation |
|---|---|
| PF3D7_0416800 | small GTP-binding protein sar1 |