Parasite plasma membrane dynamics and correlation with BTP1. (A) Schematic of the structure of SMS1 (PFF1210w). Predicted transmembrane
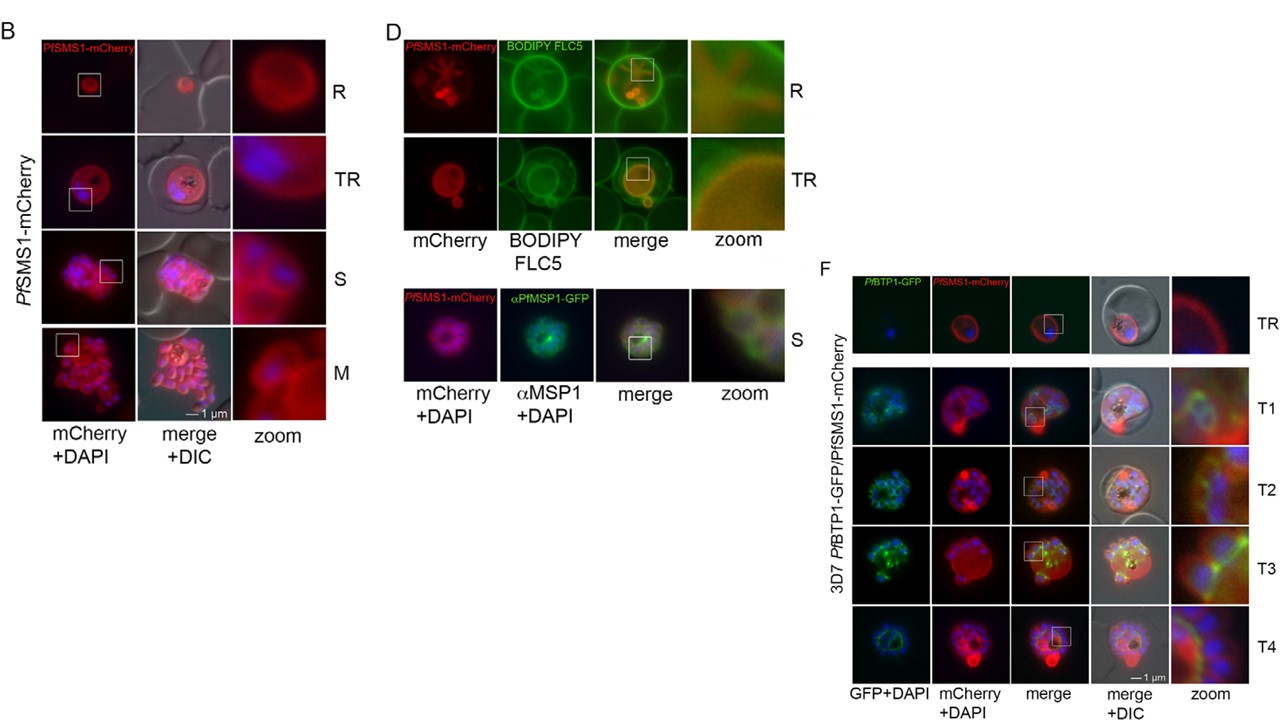
domains are indicated in blue. (B) Localization of SMS1–mCherry (red) during asexual proliferation in rings (R), trophozoites (TR), schizonts (S) and merozoites
(M). Nuclei were stained with DAPI (blue). Enlargement of selected areas are marked with a white square and referred to as ‘zoom’ (a fourfold magnification).
Please also refer to Fig. 3 and Movie 3. (C) Expression of SMS1–mCherry was analyzed by western blotting analysis using antibodies against mCherry
(α-mCherry), resulting in the detection of a fusion protein of approximately 80 kDa (calculated molecular mass 78 kDa). (D) Colocalization of SMS1–mCherry with
the membrane marker fluorophore-labeled BODIPY©-FL C5-sphingomyelin lipid (upper panels) or the plasma membrane surface protein MSP1 (lower panel;
αMSP1). (F) Colocalization of BTP1–GFP (green) with SMS1–mCherry (red) in trophozoites (TR) and during schizogony (T1–T4). Nuclei were stained with DAPI (blue). Enlargement of selected areas are marked with a white square and referred to as ‘zoom’. (G) Quantification of the colocalization analysis of BTP1–GFP with SMS1–mCherry (please refer to F). (H) Expression of BTP1–GFP and SMS1–mCherry was confirmed by western blotting analysis using antibodies against GFP and mCherry. DIC, differential interference contrast
Kono M, Heincke D, Wilcke L, Wong TW, Bruns C, Herrmann S, Spielmann T, Gilberger TW. Pellicle formation in the malaria parasite. J Cell Sci. 2016 129(4):673-80.
Other associated proteins
| PFID | Formal Annotation |
|---|---|
| PF3D7_0625000 | sphingomyelin synthase 1, putative |