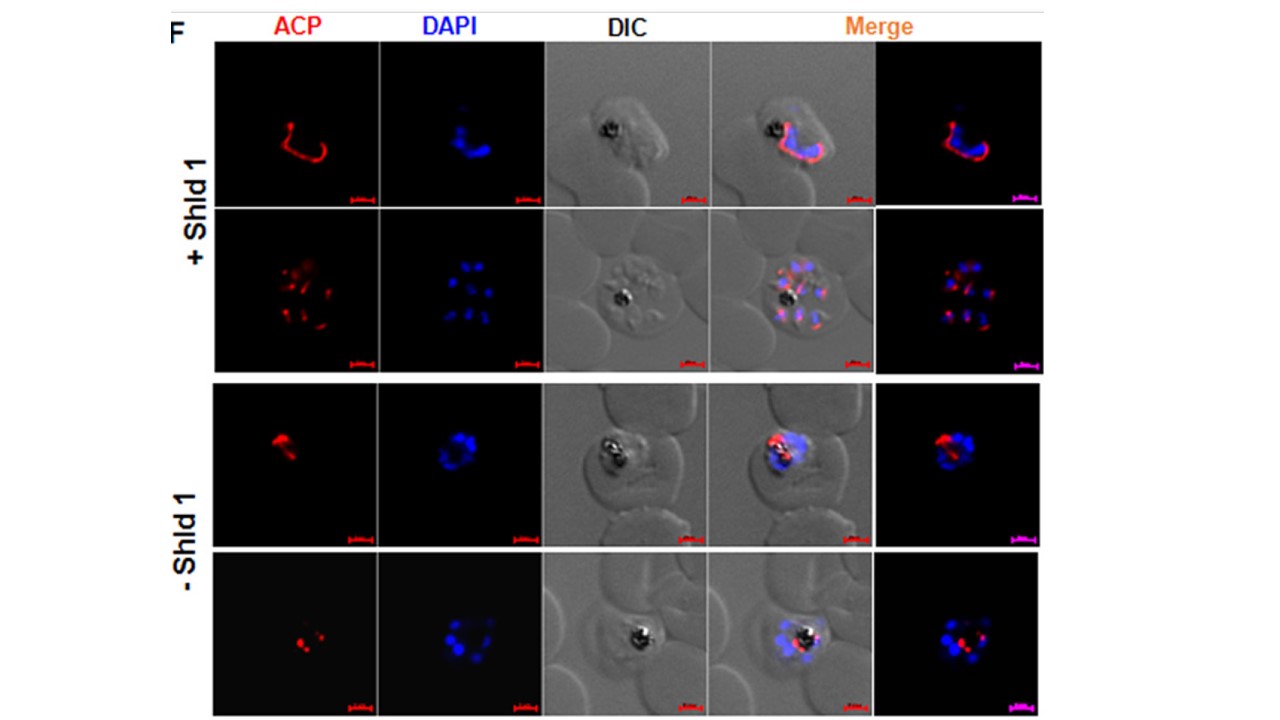
PfATG18-3HA-DD parasites were synchronized, and ring-stage parasites were cultured in the presence or absence of Shld-1. Early or late schizonts after the first, second, and third cycle (shown here) were used for IFA using an antibody against ACP, an apicoplast resident protein. In the presence of Shld-1, apicoplast branching was visible in early division and subsequently each of the newly formed merozoites received an individual apicoplast. Shld-1 removal resulted in stunted branching of ACP and impaired segregation. IFAs were performed on parasites after Shld-1 removal from PfATG18-3HA-DD parasites using an antibody against acyl carrier protein (ACP) (Fig. 3F), which are apicoplast
resident proteins. As reported earlier (24), in the wild-type situation, ACP staining revealed apicoplast branching at the onset of schizogony. Subsequently, the plastid segregated and segmented with each merozoite (F). In contrast, the apicoplast did not segregate to individual parasites in a significant number of Shld-1-deprived parasites, and branching was stunted.
Bansal P, Tripathi A, Thakur V, Mohmmed A, Sharma P. Autophagy-Related Protein ATG18 Regulates Apicoplast Biogenesis in Apicomplexan Parasites. MBio. 2017 Oct 31;8(5). pii: e01468-17.
Other associated proteins
| PFID | Formal Annotation |
|---|---|
| PF3D7_1012900 | autophagy-related protein 18 |