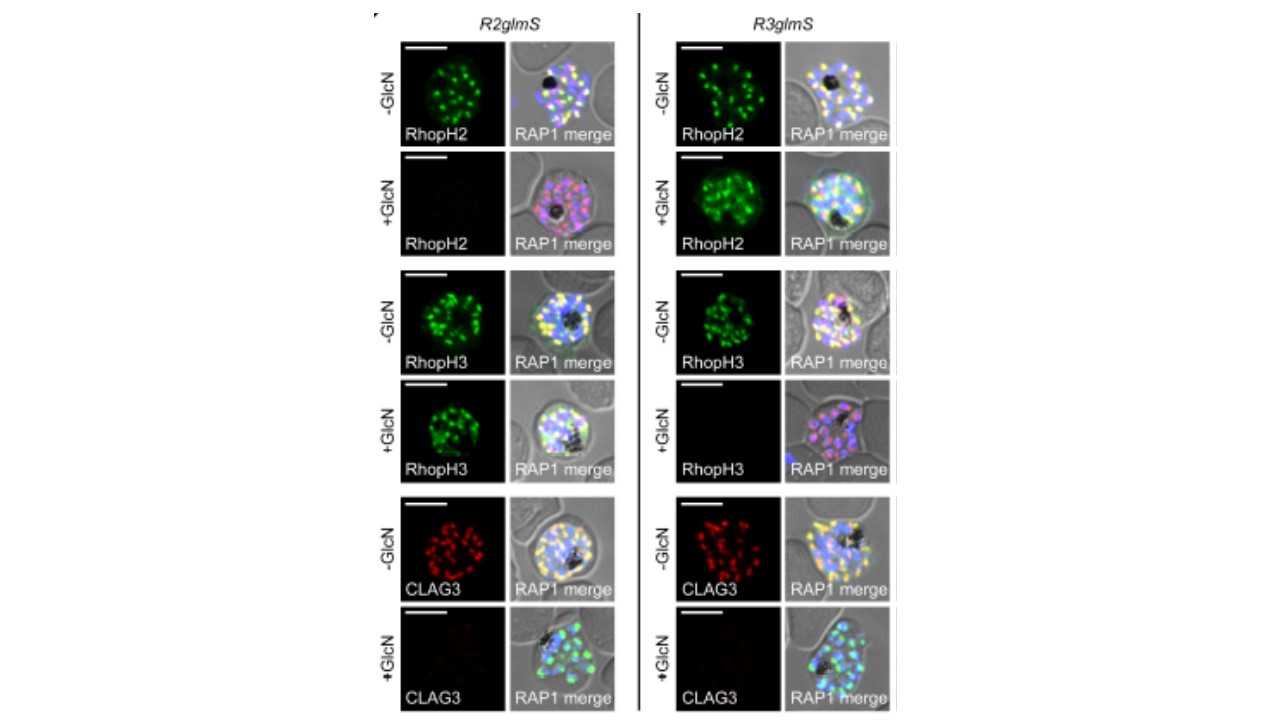
IFA of indicated proteins in R2glmS (RopH2 knockdown) and R3glmS (RopH3 knockdown) at schizont stage; colocalization with RAP1 and apical puncta in daughter merozoites indicates rhoptry trafficking. Scale bars, 5 μm. GlcN treatment of either R2glmS or R3glmS abolished not only the targeted gene product but also CLAG3, a third better-characterized member of the RhopH complex. Indirect immunofluorescence microscopy confirmed this surprising finding and provided additional insights. While RhopH2 was still detected in the R3glmS knockdown, it did not fully colocalize with RAP1 but instead had a more diffuse distribution; this suggests RhopH2 may be retained in the parasite endoplasmic reticulum if it fails to interact with RhopH3. In contrast, RhopH3 appeared to traffic normally in
GlcN-treated R2glmS parasites, as indicated by apical colocalization with RAP1 in
merozoites. Thus, CLAG3, RhopH2 and RhopH3 assemble into a complex either during or immediately after translation.
Ito D, Schureck MA, Desai SA. An essential dual-function complex mediates erythrocyte invasion and channel-mediated nutrient uptake in malaria parasites. Elife. 2017 Feb 21;6.
Other associated proteins
| PFID | Formal Annotation |
|---|---|
| PF3D7_0302200 | cytoadherence linked asexual protein 3.2 |
| PF3D7_0905400 | high molecular weight rhoptry protein 3 |
| PF3D7_1410400 | rhoptry-associated protein 1 |