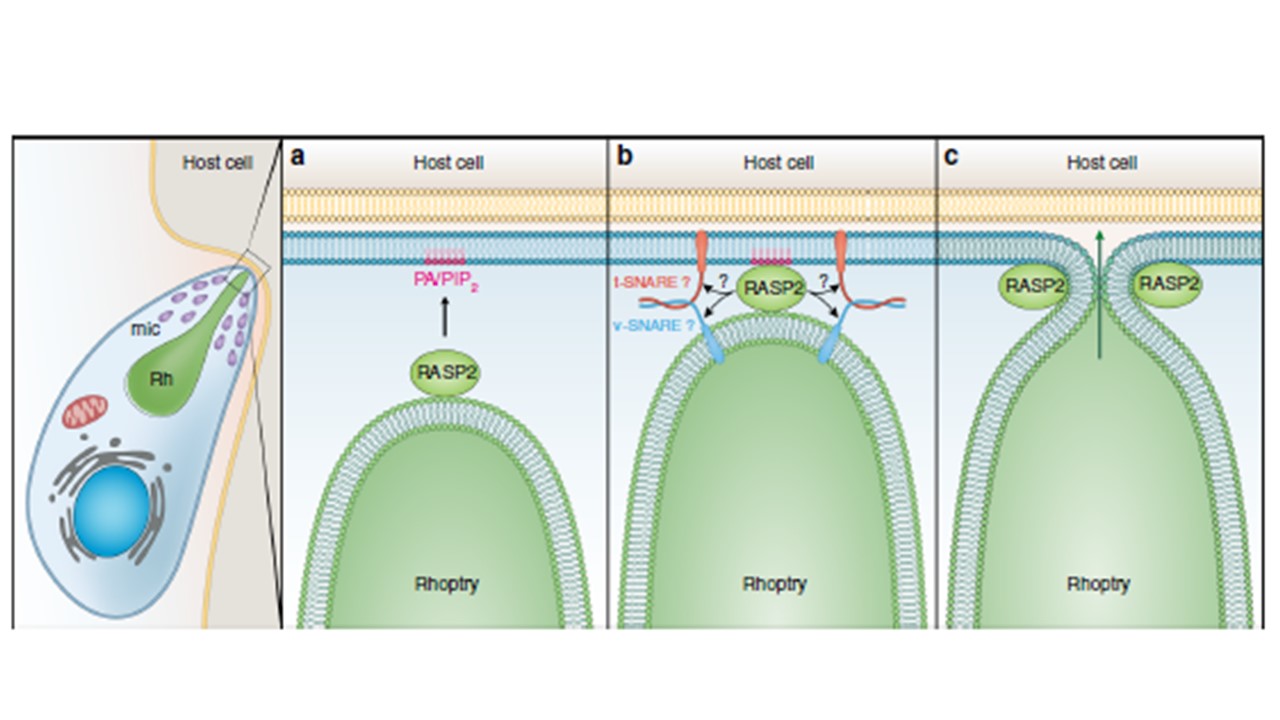
Model of RASP2 rhoptry docking/priming function in apicomplexan parasites. Upon response to an unknown signal, RASP2 binds to phospholipids (PA/PIP2) (a). This promotes association of the rhoptry with the parasite plasma membrane to initiate the assembly of the docking/primModel of RASP2 rhoptry docking/priming function in apicomplexan parasites. Upon response to an unknown signal, RASP2 binds to phospholipids (PA/PIP2) (a). This promotes association of the rhoptry with the parasite plasma membrane to initiate the assembly of the docking/priming machinery of the rhoptry (b), which results in membrane fusion, an essential step before the release of the rhoptry contents into the host cell (c). Suarez C, Lentini G, Ramaswamy R, Maynadier M, Aquilini E, Berry-Sterkers L, Cipriano M, Chen AL, Bradley P, Striepen B, Boulanger MJ, Lebrun M. A lipid-binding protein mediates rhoptry discharge and invasion in Plasmodium falciparum and Toxoplasma gondii parasites. Nat Commun. 2019 10(1):4041. PMID: 31492901;
Other associated proteins
| PFID | Formal Annotation |
|---|---|
| PF3D7_0314100 | vesicle transport v-SNARE protein, putative |
| PF3D7_0530100 | SNARE protein, putative |
| PF3D7_0826600 | SNARE protein, putative |
| PF3D7_0910600 | SNARE protein |
| PF3D7_1236000 | vesicle transport v-SNARE protein VTI1, putative |
| PF3D7_1320500 | SNARE protein, putative |
| PF3D7_1324700 | SNARE protein, putative |
| PF3D7_1448600 | SNARE protein, putative |