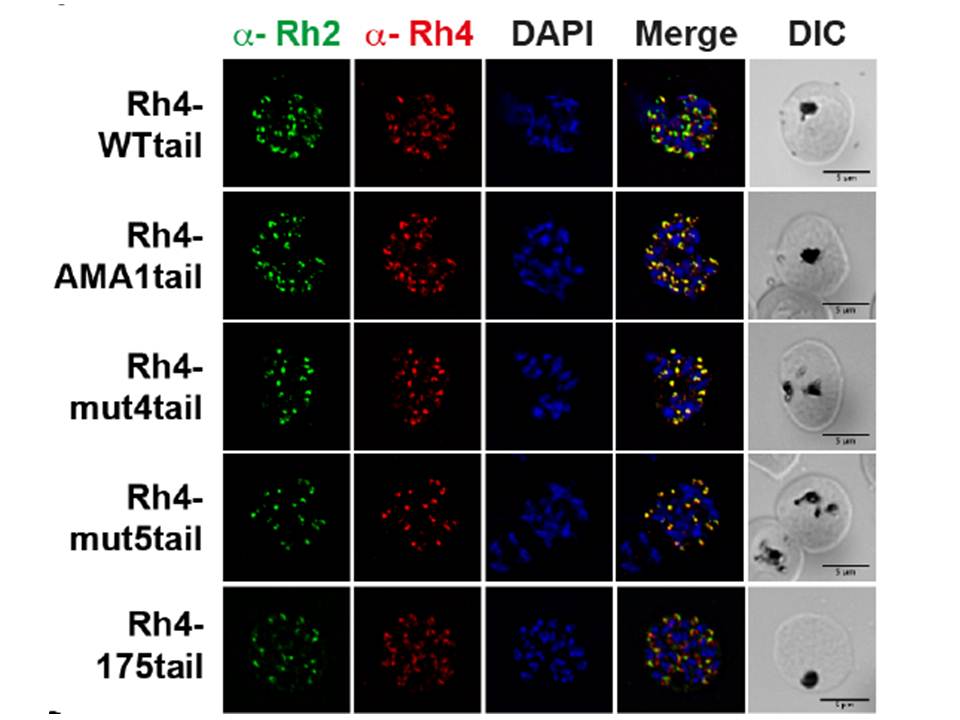
Specific amino acid residues in the cytoplasmic domain are essential for PfRh4 function. Localization of PfRh4 and PfRh2 in Rh4-WT tail, RH4-AMA1 tail (PfRh4 tail was replaced with the cytoplasmic sequence of AMA1), Rh4-mut4tail ) contains the mutations S1667A, S1674A, Y1680A and Y1684A) and RH4-mut5tail (contains similar mutations with an addition mutation at S1652A within the PfRh4 cytoplasmic tail) lines as detected using anti-PfRh4 monoclonal and anti-PfRh2 polyclonal antibodies. Parasite nuclei were stained with DAPI. Rh4-mut4 tail and Rh4-mut5 tail expressed PfRh4 and all showed an apical localization similar to rhoptry protein PfRh2
Tham WH, Lim NT, Weiss GE, Lopaticki S, Ansell BR, Bird M, Lucet I, Dorin-Semblat D, Doerig C, Gilson PR, Crabb BS, Cowman AF. Plasmodium falciparum Adhesins Play an Essential Role in Signalling and Activation of Invasion into Human Erythrocytes. PLoS Pathog. 2015 Dec 22;11(12):e1005343.
Other associated proteins
| PFID | Formal Annotation |
|---|---|
| PF3D7_0424200 | reticulocyte binding protein homologue 4 |
| PF3D7_1335400 | reticulocyte binding protein 2 homologue a |