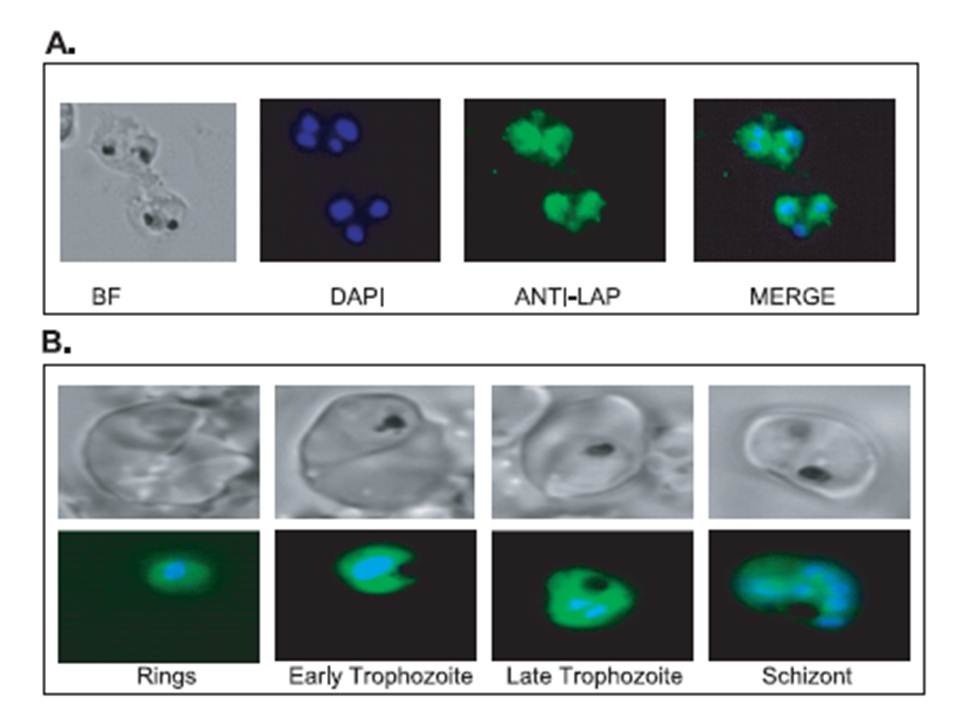
Localization of the P. falciparum M17 leucyl aminopeptidase in the intra-erythrocytic parasites. A, immunofluorescence assays were carried out using air-dried P. falciparum-infected red blood cells fixed with acetone. Cells were probed with mouse anti-M17 peptide (1/250) and then Cy2-conjugated goat anti-mouse antibodies. B, transgenic P. falciparum strain D10 parasites expressing the complete M17 leucyl aminopeptidase linked to green fluorescent protein were visualized in a live fluorescence assay. The parasite nuclei were visualized with Hoechst dye (0.5 mg/ml). Samples were viewed on an Axioscope 2 Mot + (Zeiss) equipped with a Zeiss 63x/1.4 Plan Apochromat lens. Images were captured with an Axiocam MRm camera (Zeiss) using Axiovision AC software (Zeiss). BF, bright field; DAPI, 4,6-diamidino-2-phenylindole.
Stack CM, Lowther J, Cunningham E, Donnelly S, Gardiner DL, Trenholme KR, Skinner-Adams TS, Teuscher F, Grembecka J, Mucha A, Kafarski P, Lua L, Bell A, Dalton JP. Characterization of the Plasmodium falciparum M17 leucyl aminopeptidase. A protease involved in amino acid regulation with potential for antimalarial drug development. J Biol Chem. 2007 282:2069-2080.