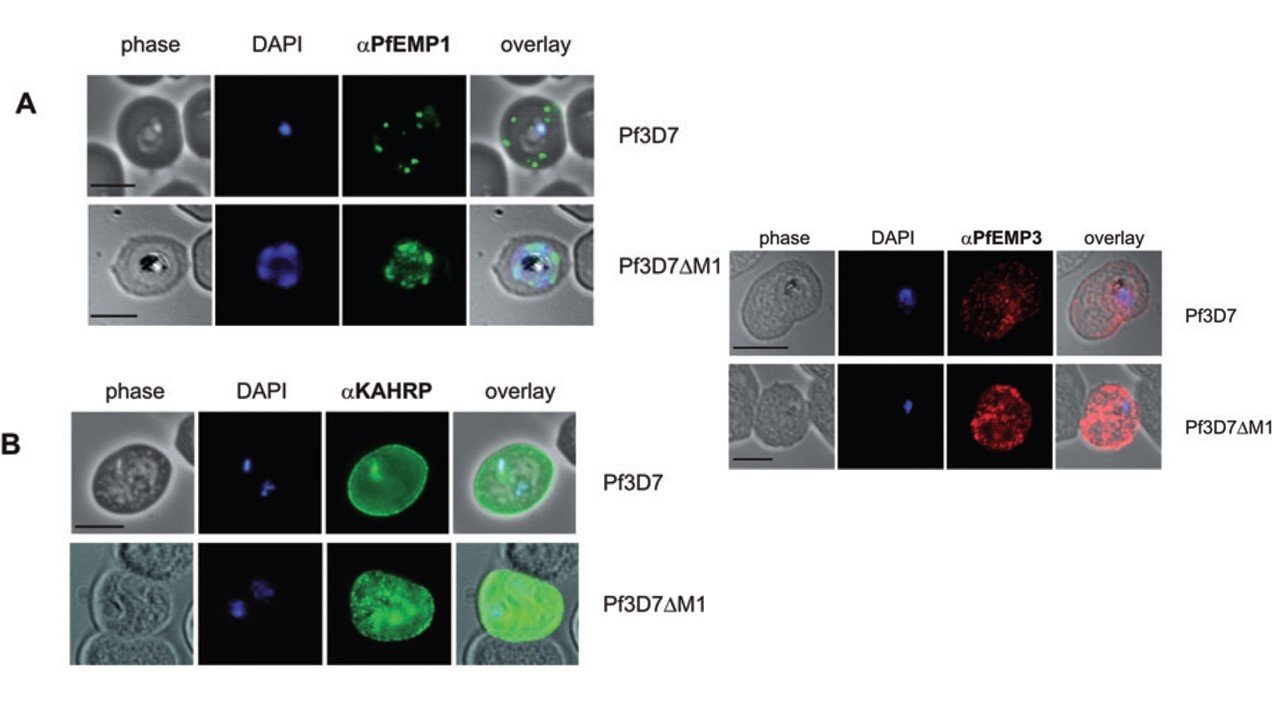
Immunofluorescence microscopy of exported proteins. Smears of RBCs infected with parental wild-type 3D7 (top row) and Pf3D7DM1 (bottom row) were probed with antibodies recognizing proteins exported to the RBC membrane via Maurer’s clefts. Scale bar = 5 mm. In the parent line, PfEMP1 showed a characteristic accumulation at the Maurer’s clefts. In contrast, PfEMP1 accumulated within the confines of the parasite plasma membrane/PVM in Pf3D7DM1-infected RBCs. the locations of proteins that are known to transiently associate with the Maurer’s clefts before associating with the RBC membrane (KAHRP and PfEMP3). KAHRP displayed a fluorescence profile consistent with a location at the RBC membrane in both wild type and Pf3D7DM1 parasites (B), indicating correct trafficking to knob structures. PfEMP3 showed a heterogeneous distribution over the host cell (C) in both lines consistent with previous reports, showing that this protein is correctly exported to the host cell cytoskeleton. Thus, both KAHRP and PfEMP3 appear to be exported independently of MAHRP1
Spycher C, Rug M, Pachlatko E, Hanssen E, Ferguson D, Cowman AF, Tilley L, Beck HP. The Maurer's cleft protein MAHRP1 is essential for trafficking of PfEMP1 to the surface of Plasmodium falciparum-infected erythrocytes. Mol Microbiol. 2008 68(5):1300-14.
Other associated proteins
| PFID | Formal Annotation |
|---|---|
| PF3D7_0202000 | knob-associated histidine-rich protein |
| PfEMP1 | PfEMP1 |