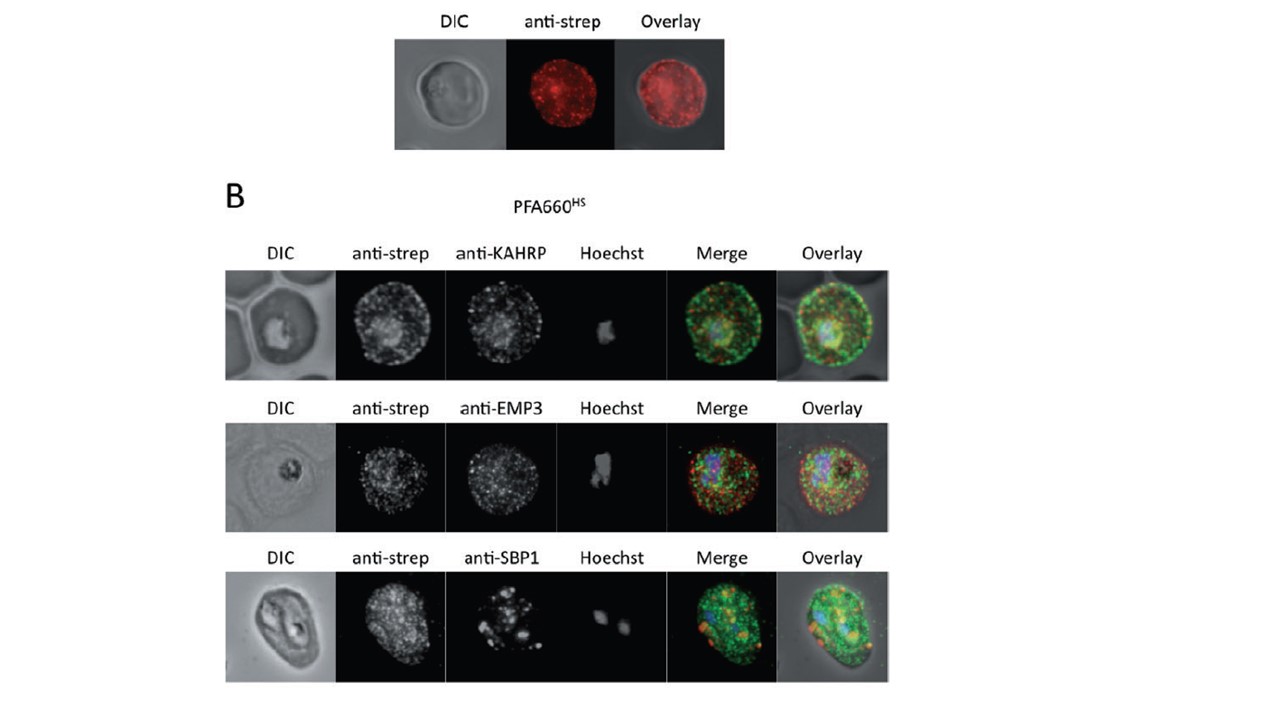
Co-immunofluorescence microscopy of HA-strep transfectant lines. A. Immunofluorescence analysis on erythrocytes infected with PFE55HS using anti-strep antibodies. Red, anti-strep. B. Co-immunofluorescence analysis on erythrocytes infected with PFA660HS, using antisera against strep tag (all panels) and either KAHRP (upper panel), PfEMP3 (middle panel) or SBP1 (lower panel). Fluorescence channels are shown individually in black/white for highest contrast. In merge image green, anti-strep; red, KAHRP (upper panel), PfEMP3 (middle panel) or SBP1 (lower panel); blue, Hoechst. No colocalization of GFP and KAHRP/PfEMP3 or SBP1 signals can be observed. Immunofluorescence colocalization experiments on the PFA660–HS cell line again revealed no colocalization between this fusion protein, and Maurer’s clefts markers KAHRP, PfEMP3 and PfSBP1 (B). These results further verify that the structures labelled by tagged PFE55/PFA660 represent novel extra-parasitic structures in the P. falciparum-infected erythrocyte, distinct in protein composition from the Maurer’s clefts.
Külzer S, Rug M, Brinkmann K, Cannon P, Cowman A, Lingelbach K, Blatch GL, Maier AG, Przyborski JM. Parasite-encoded Hsp40 proteins define novel mobile structures in the cytosol of the P. falciparum-infected erythrocyte. Cell Microbiol. 2010 12(10):1398-420.
Other associated proteins
| PFID | Formal Annotation |
|---|---|
| PF3D7_0113700 | heat shock protein 40, type II |
| PF3D7_0202000 | knob-associated histidine-rich protein |
| PF3D7_0501100 | co-chaperone j domain protein jdp |
| PF3D7_0501300 | skeleton-binding protein 1 |