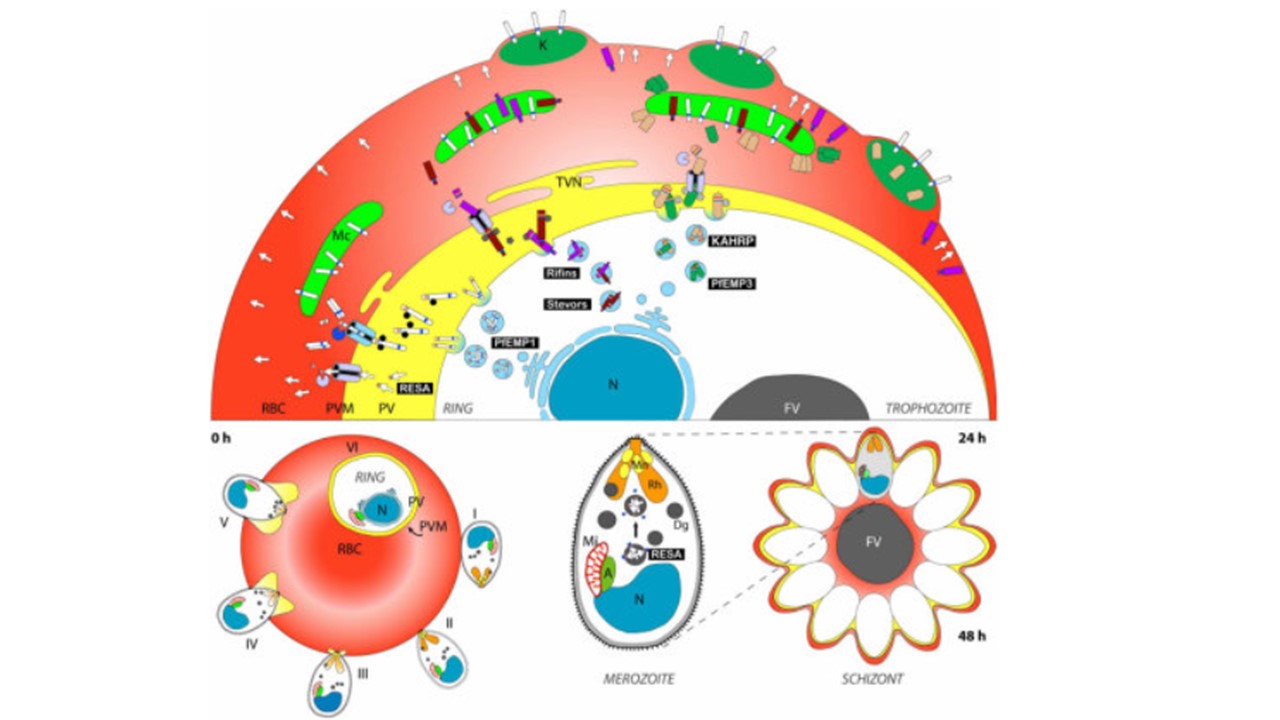
Approximately 400 parasite proteins have a predicted PEXEL motif that is expected to direct export to the RBC cytosol. Many of these may play roles in trafficking of PfEMP1 to the RBC surface or modifying the RBC membrane in other ways to suit the parasite’s needs. Plasmodium prepares for the invasion and remodelling process of a new host cell in late asexual stages (schizont stage) by transporting crucial molecules to the three prototypic apicomplexan organelles (the microneme [Mn], the rhoptry [Rh], and the dense granule [Dg]) located close to the apical end of the parasite. While microneme proteins are mainly involved in initiating invasion (stages I–III), rhoptry and dense granule proteins are implicated in establishment of the parasite in the newly invaded host cell (stages III–VI). RESA (arrows), for
example, is targeted to the dense granules in late schizogony, released into the parasitophorous vacuole (PV) after invasion, and subsequently exported into the red blood cell (RBC), where it associates with the red cell cytoskeleton to stabilize the newly invaded RBC membrane. The presence of a PEXEL motif in RESA implies the establishment of the corresponding translocation machinery very early after formation of the PVM. This allows the virulence factor PfEMP1 to be translocated already in early ring stages. It then appears to be transported as a soluble complex and inserted into the Maurer’s cleft (MC) membrane. In trophozoite stages, knob components such as KAHRP and PfEMP3 are exported and travel as large complexes through the RBC cytosol until they transiently associate with components on the MC. Further transport of KAHRP, PfEMP3, and PfEMP1 leads to knob formation and surface exposition of PfEMP1. It is not known whether Rifin and Stevor proteins are also trafficked as soluble complexes in the RBC cytosol or whether there is vesicular transport between the PVM, MCs, and possibly the RBC membrane. Mi, mitochondrion; A, apicoplast. Marti M, Baum J, Rug M, Tilley L, Cowman AF. Signal-mediated export of proteins from the malaria parasite to the host erythrocyte. J Cell Biol. 2005 171(4):587-92.