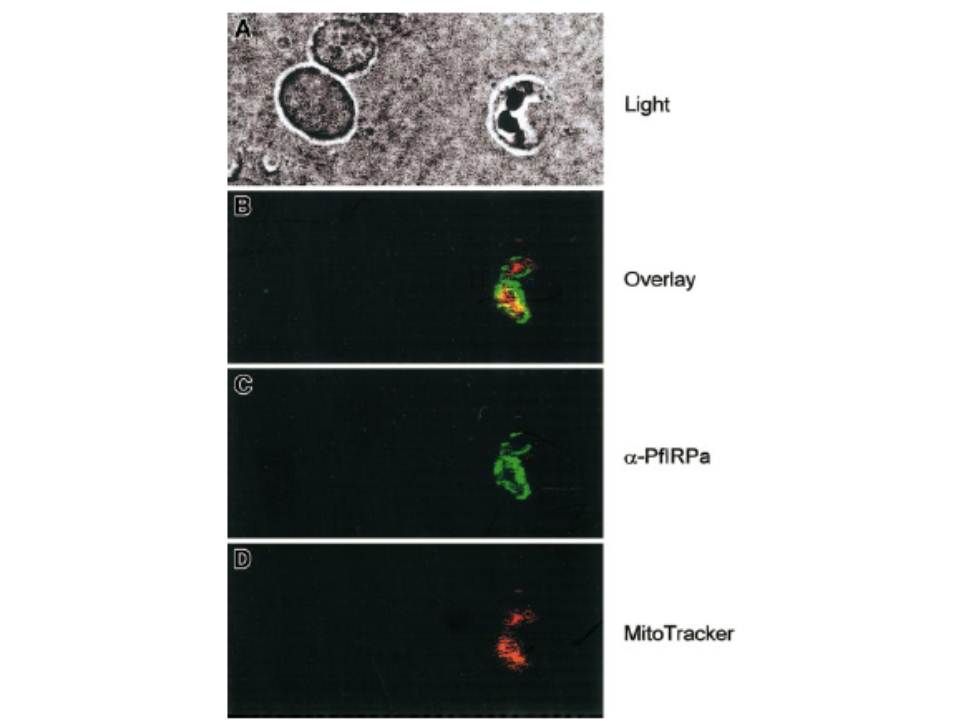
Visualization of PfIRPa via indirect immunofluorescence probing. The suspension of infected erythrocytes was first loaded with 50 nmoles MitoTracker for 30 minutes. The suspension was washed and fixed with 4% paraformaldehyde, 0.05% glutaraldehyde. The cells were allowed to adhere and to dry to poly-L-lysine–coated microscopic slides. The slides were washed in PBS-Tween (0.01%) and PBS. Nonspecific epitopes of cells were quenched with 5% BSA in PBS, washed, and probed with a primary antibody (rabbit polyclonal 3950) followed by a secondary antibody (goat antirabbit, alexa488). The slides were washed and mounted in the presence of VectaShield liquid, before taking fluorescence images. The images were taken at the excitation wavelengths of 488 nm for alexa488 and at the 680 nm for MitoTracker. Our immunofluorescence data further support the conclusion that PfIRPa is a cytosolic malarial protein, and it is not present in the mitochondrion of the parasite, at least at the trophozoite stage.
Loyevsky M, LaVaute T, Allerson CR, Stearman R, Kassim OO, Cooperman S, Gordeuk VR, Rouault TA. An IRP-like protein from Plasmodium falciparum binds to a mammalian iron-responsive element. Blood. 2001 98:2555-62.