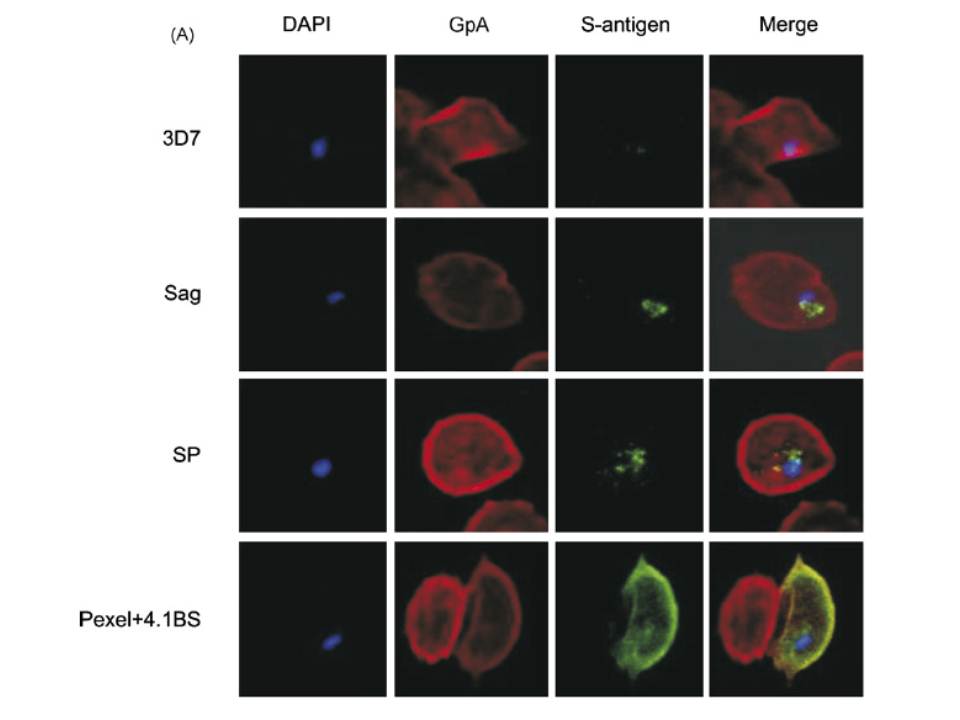
A mouse antibody to RBC membrane protein Glycophorin A (GpA) was used as a positive control for the IFA experiments. The images show that the proteins expressed by control line (3D7 Sag) which contains S-antigen alone (with its endogenous signal peptide) remained confined within the parasite or the PV, and was not exported into the RBC cytosol. The protein expressed by 3D7 SP (expressing exon 1 of MESA fused to S-antigen) was also retained within the PV, indicating that the embedded signal peptide of MESA is not sufficient to transport the protein across the PVM. The image for 3D7 Pexel+4.1BS, which contains the putative Pexel and the 4.1R-binding site, shows that the protein was trafficked outside the parasite and appeared to co-localise with GpA at the RBC membrane. Transfectant parasite line 3D7 D4.1BS (lacking the 4.1R-binding site) showed a vastly different staining pattern to that of 3D7 Pexel+4.1BS. It appeared that the D4.1BS/S-antigen chimera was being transported outside the parasite, indicating that the Pexel is functional and is the minimum sequence required for passage through the PVM into the RBC cytosol.
Black CG, Proellocks NI, Kats LM, Cooke BM, Mohandas N, Coppel RL. In vivo studies support the role of trafficking and cytoskeletal-binding motifs in the interaction of MESA with the membrane skeleton of Plasmodium falciparum-infected red blood cells. Mol Biochem Parasitol. 2008 160:143-7. Copyright Elsevier 2009.