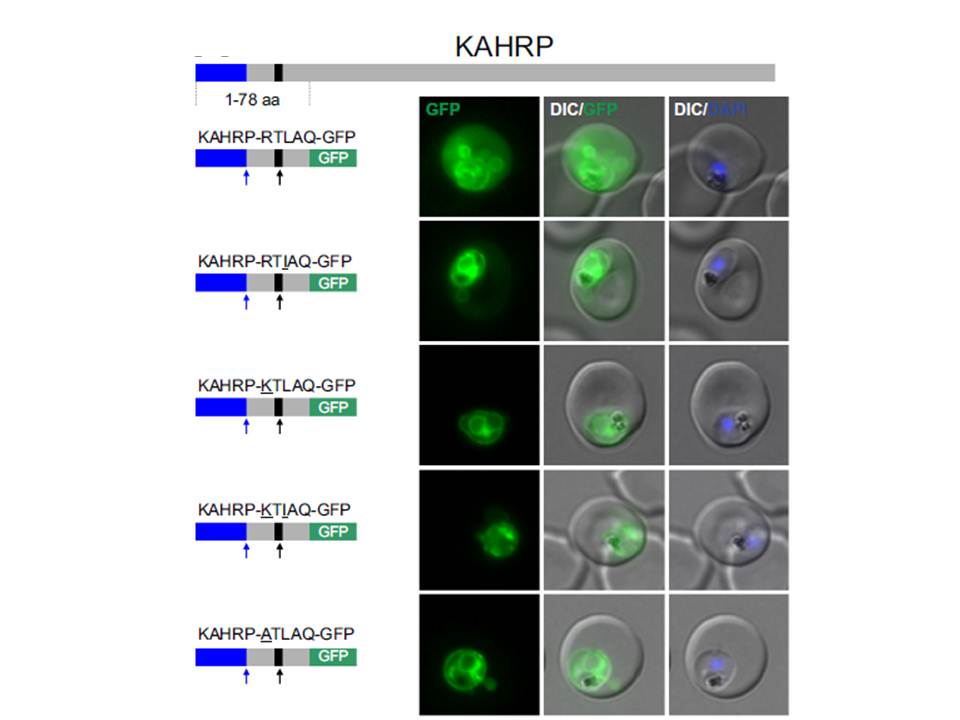
Non-canonical PEXEL/HTs are not functional in a KAHRP reporter. A. Fluorescence microscopy. The structure of full-length KAHRP is indicated at the top; the schematic structure of the reporter proteins is depicted on the left of representative images of iRBCs. The ER-signal is shaded blue, the (non-) canonical PEXEL/HT is shaded black and GFP is shaded green. Putative cleavage sites are indicated by an arrow (blue: SP cleavage site; black: Plasmepsin V cleavage site. Using a truncated KAHRP reporter. Fluorescence microscopy analyses indicated that KAHRPRTLAQ-GFP and KAHRP-RTIAQ-GFP were efficiently exported . Variants with alternative amino acids at position 1 were inefficiently exported as exemplified by KAHRP-KTLAQ-GFP, KAHRP-KTIAQGFP and the control reporter KAHRP-ATLAQ-GFP.
Schulze J, Kwiatkowski M, Borner J, Schlüter H, Bruchhaus I, Burmester T, Spielmann T, Pick C. The Plasmodium falciparum exportome contains non-canonical PEXEL/HT proteins. Mol Microbiol. 2015 Apr 8. [Epub ahead of print]