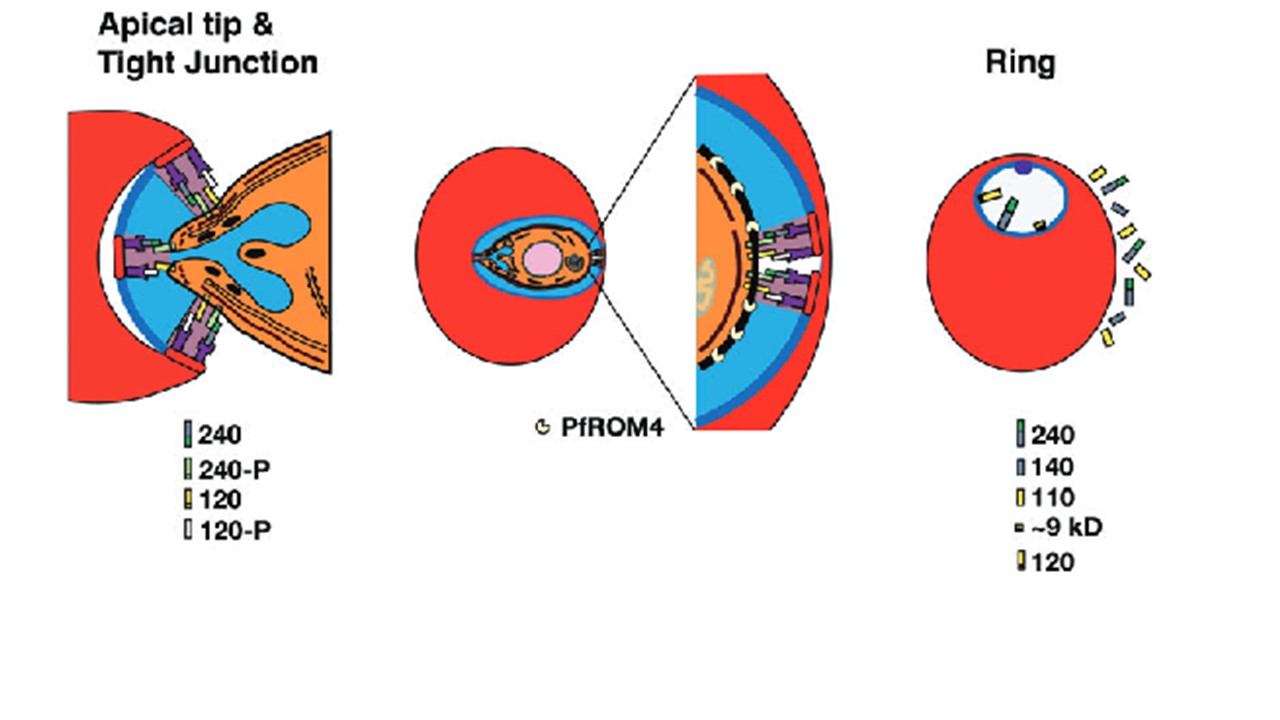
A model for invasion incorporating the roles and ultimate locations of various PfRh1 processed products. Once an erythrocyte has been targeted for invasion, proteins of the EBL and PfRH families, already at the apical tip of the merozoite, begin the invasion process. As PfRh1 is already processed to 120 and 240 kDa products prior to invasion and as both products are at the tight junction, the existence of both a 240 partner protein (240-P) and a 120 partner protein (120-P) must be envisaged. The 240-P protein most likely has a transmembrane domain in order to anchor the Rh1-240 binding domain to the merozoite surface while the 120-P protein most likely binds an erythrocyte receptor and is anchored to the merozoite surface by the Rh1-120 protein. The rhoptry contents together with components of the erythrocyte membrane fuse to form the nascent parasitophorous vacuolar membrane shown in a darker blue colour. While the majority of the Rh1-240and Rh1-120 proteins remain at the apical tip, a smaller proportion are incorporated into the tight junction, which moves to the posterior of the merozoite by connections to the underlying actin–myosin motor complex. Once at the posterior the components of the tight junction are cleaved by intramembrane rhomboid proteases such as PfROM4. The right panel shows the final location of the various PfRh1 processed products either within the newly developing Ring or in culture supernatant. Triglia T, Tham WH, Hodder A, Cowman AF. Reticulocyte binding protein homologues are key adhesins during erythrocyte invasion by Plasmodium falciparum. Cell Microbiol. 2009 PMID: 19614665
Other associated proteins
| PFID | Formal Annotation |
|---|---|
| PF3D7_0506900 | rhomboid protease ROM4 |