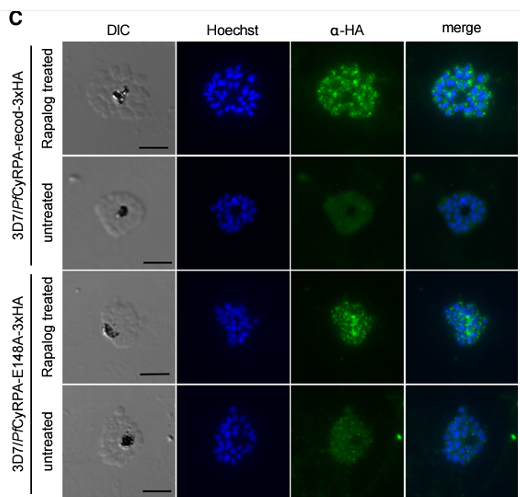
Anti-HA IFA experiments in fixed 3D7/PfCyRPA-recodonized-3xHA (two top rows) and 3D7/PfCyRPA-E148A-3xHA (two bottom rows), which were treated or untreated with Rapalog. DNA was counterstained using Hoechst. Scale bar: 5 mm. PfCyRPA is a lectin targeting glycans terminating with a2-6-linked N-acetylneuraminic acid (Neu5Ac). PfCyRPA has a >50-fold binding preference for human, a2-6-linked Neu5Ac over non-human, a2-6-linked N-glycolylneuraminic acid. PfCyRPA lectin sites were predicted by molecular modeling and validated by mutagenesis studies. Transgenic parasite lines expressing endogenous PfCyRPA with single amino acid exchange mutants indicated that the lectin activity of PfCyRPA has an important role in parasite invasion. Blocking PfCyRPA lectin activity with small molecules or with lectin-site-specific monoclonal antibodies can inhibit blood-stage parasite multiplication. Therefore, targeting PfCyRPA lectin activity with drugs, immunotherapy, or a vaccine-primed immune response is a promising strategy to prevent and treat malaria. Day CJ, Favuzza P, Bielfeld S, Haselhorst T, Seefeldt L, Hauser J, Shewell LK, Flueck C, Poole J, Jen FE, Schäfer A, Dangy JP, Gilberger TW, França CT, Duraisingh MT, Tamborrini M, Brancucci NMB, Grüring C, Filarsky M, Jennings MP, Pluschke G. The essential malaria protein PfCyRPA targets glycans to invade erythrocytes. Cell Rep. 2024 43(4):114012