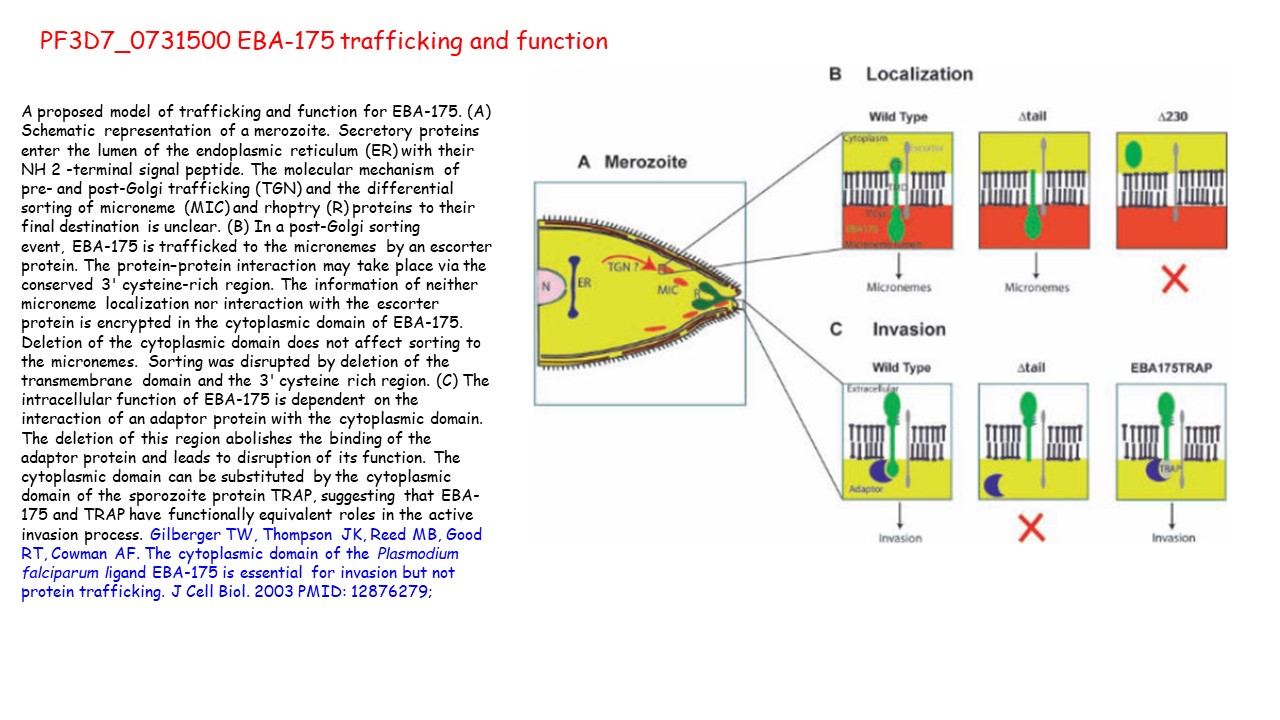
A proposed model of trafficking and function for EBA-175. (A) Schematic representation of a merozoite. Secretory proteins enter the lumen of the endoplasmic reticulum (ER) with their NH 2 -terminal signal peptide. The molecular mechanism of pre- and post-Golgi trafficking (TGN) and the differential
sorting of microneme (MIC) and rhoptry (R) proteins to their final destination is unclear. (B) In a post-Golgi sorting event, EBA-175 is trafficked to the micronemes by an escorter protein. The protein–protein interaction may take place via the conserved 3' cysteine-rich region. The information of neither
microneme localization nor interaction with the escorter protein is encrypted in the cytoplasmic domain of EBA-175. Deletion of the cytoplasmic domain does not affect sorting to the micronemes. Sorting was disrupted by deletion of the
transmembrane domain and the 3' cysteine rich region. (C) The intracellular function of EBA-175 is dependent on the interaction of an adaptor protein with the cytoplasmic domain. The deletion of this region abolishes the binding of the adaptor protein and leads to disruption of its function. The cytoplasmic domain can be substituted by the cytoplasmic domain of the sporozoite protein TRAP, suggesting that EBA-175 and TRAP have functionally equivalent roles in the active invasion process. Gilberger TW, Thompson JK, Reed MB, Good RT, Cowman AF. The cytoplasmic domain of the Plasmodium falciparum ligand EBA-175 is essential for invasion but not protein trafficking. J Cell Biol. 2003