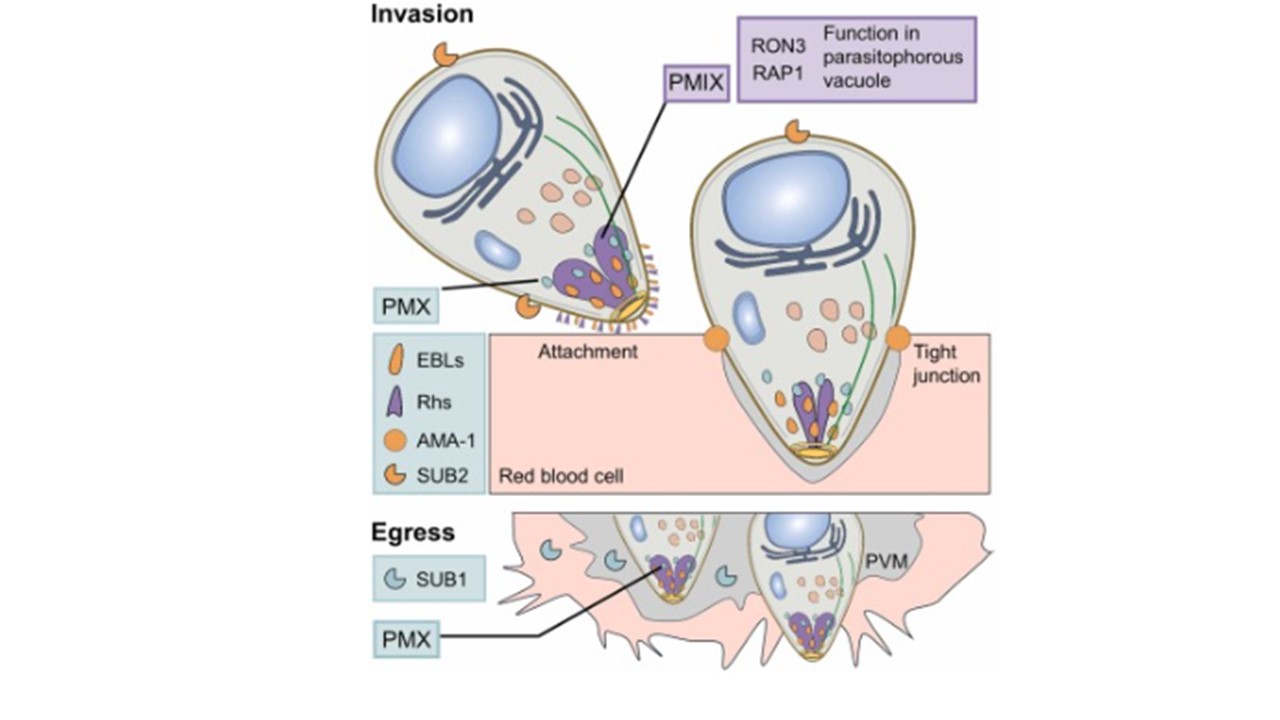
Exoneme-residing Plasmepsin X (PMX) is responsible for processing proteins and proteases involved in the merozoite invasion of host erythrocytes. PMX cleaves microneme and rhoptry proteins that are part of the surface adhesins group, including erythrocyte-binding ligands (EBLs) and reticulocyte-binding homologues (Rhs). These adhesins play a crucial role during the initial merozoite attachment to erythrocytes. Additionally, PMX activates a membrane-bound serine protease, Subtilisin 2 (SUB2), which mediates the shedding of apical membrane antigen 1 (AMA-1). AMA-1 forms a tight junction with rhoptry neck proteins, facilitating the parasite's propulsion into the erythrocyte. Plasmepsin IX (PMIX), stored in rhoptries, cleaves rhoptry neck protein 3 (RON3) and rhoptry-associated protein 1 (RAP1), which are vital for ring-stage development within the parasitophorous vacuole. PMX is also involved in egress by activating Subtilisin 1 (SUB1), which processes substrates that enable the rupture of both the parasitophorous vacuole (PVM) and the erythrocyte surface membranes. Sojka D, Šnebergerová P. Advances in protease inhibition-based chemotherapy: A decade of insights from Malaria research. Adv Parasitol. 2024;126:205-227.
Other associated proteins
| PFID | Formal Annotation |
|---|---|
| PF3D7_0105200 | RAP01 |
| PF3D7_0507500 | subtilisin-like protease 1 |
| PF3D7_1136900 | subtilisin-like protease 2 |
| PF3D7_1252100 | rhoptry neck protein 3 |
| PF3D7_1430200 | plasmepsin IX |