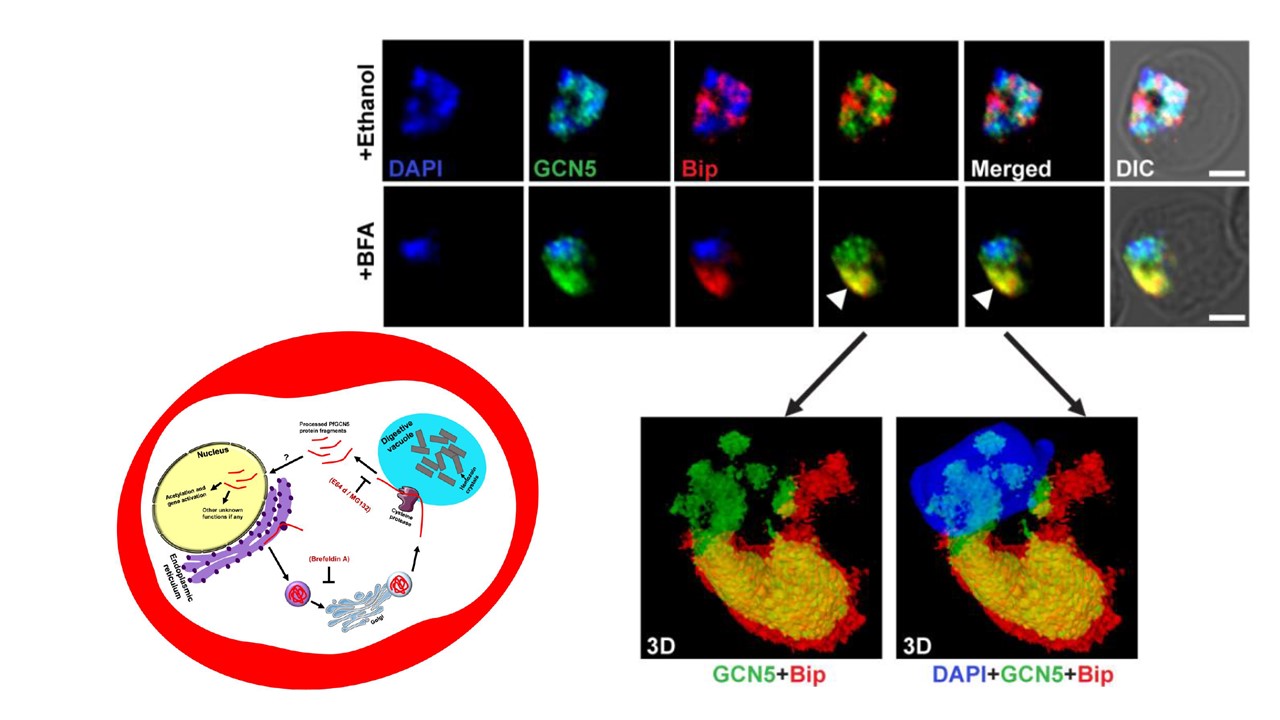
PfGCN5 protein gets stabilized and redistributed to the ER compartment upon treatment with brefeldin A (BFA). Effect of BFA on the localization of PfGCN5 protein in fixed parasites. ER-resident protein Bip was used as an ER marker. Immunofluorescence analysis shows different distribution of PfGCN5 and Bip in ethanol treated cells (top panel). BFA treated parasites show significant co-localization of the above two proteins (indicated by the white arrow; middle panel). The bottom panel shows the 3D reconstruction of the selected images from the middle panel. Left bottom: Proposed model for the processing event of PfGCN5 in the parasite. The full-length PfGCN5 protein possibly gets translated on the ER and transport to the digestive vacuole via Golgi, in the secretory vesicles. This ER-Golgi transport process can be blocked by brefeldin A (BFA). A cysteine protease is either present on the food vacuole membrane or in close vicinity of the food vacuole process the full-length protein in multiple specific fragments, containing some part of C-terminal region of PfGCN5. These fragments enter the nucleus via an undefined route. In the parasite nucleus, these PfGCN5 fragments acetylates histone H3 or possibly some other proteins and regulates multiple cellular functions.
Bhowmick K, Tehlan A, Verma S, Sudhakar R, Kaur I, Sijwali PS, Krishnamachari A, Dhar SK. Plasmodium falciparum GCN5 acetyltransferase follows a novel proteolytic processing pathway essential for its function. J Cell Sci. 2019 Dec 20. pii: jcs.236489.
Other associated proteins
| PFID | Formal Annotation |
|---|---|
| PF3D7_0917900 | PfHsp70-2 |