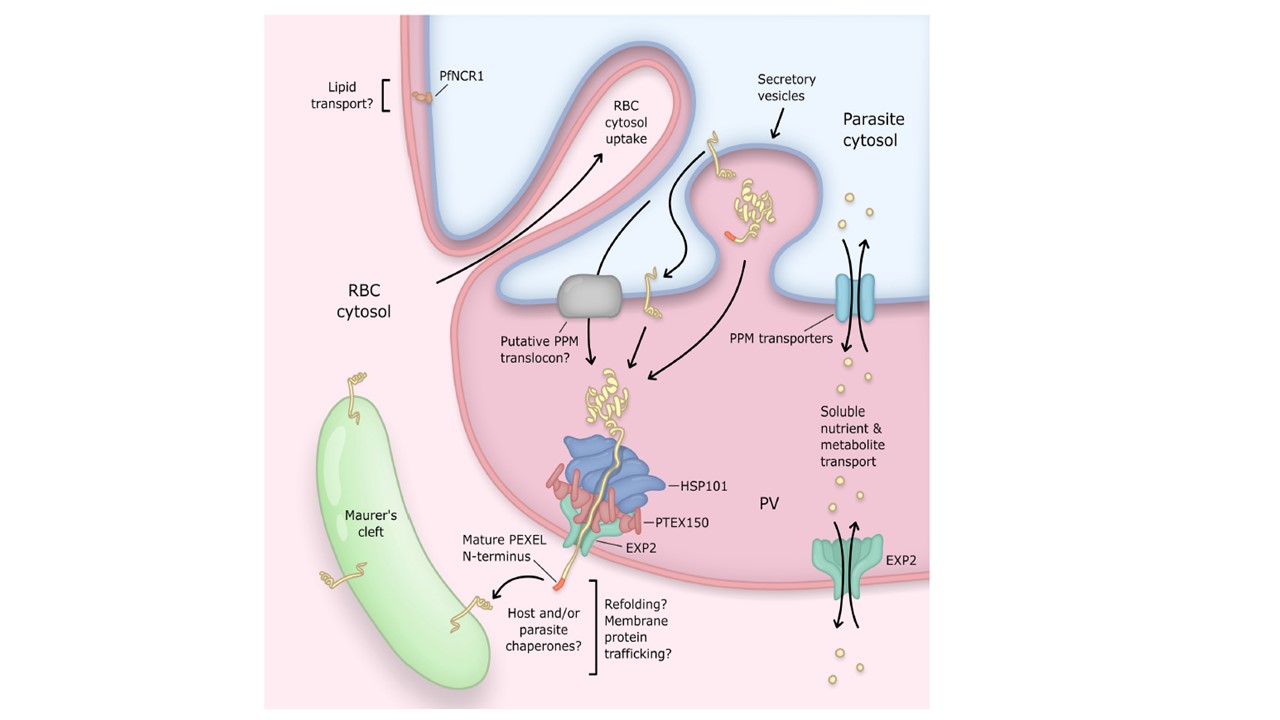
Transport activities at the PV of the blood-stage malaria parasite. The PV is the principal host–parasite interface and the site of several distinct transport activities mediating exchange of proteins and small molecules between the parasite and host compartments. The PV is compartmentalized into regions of close apposition between PVM–PPM (top left) and regions with greater luminal space (bottom right). Machinery involved in protein export and transport of solutes localizes to the latter regions. Parasite effector proteins destined for export into the host cell are delivered to the PV by secretory vesicles and then translocated across the PVM by the PTEX. Most exported proteins contain a PEXEL motif which is processed by the ER-localized aspartic protease Plasmepsin 5 to license export. Soluble
exported proteins can be directly accessed by the PTEX AAA+ unfoldase HSP101 in the PV lumen, while exported integral membrane proteins are first incorporated into the PPM and then require a translocation step for PPM extraction that appears to involve unfolding power additional to HSP101. HSP101 is coupled to the membrane-spanning EXP2 pore via a flange-shaped adaptor known as PTEX150. Mechanisms for effector refolding, trafficking, and membrane insertion beyond the PVM are largely unknown and likely involve host and/or exported parasite chaperones. The EXP2 pore also serves a secondary role to transport small molecules across the PVM, likely independent of the PTEX complex. PfNCR1, a protein similar to the NPC1 protein involved in cholesterol egress from late endosomes, localizes to regions of close PVM–PPMapposition, suggesting that these may be sites of lipid transport. Parasites also endocytose large amounts of the erythrocyte cytosol through cytosomal invaginations of the PVM–PPM. ER, endoplasmic reticulum; EXP2, exported protein 2; HSP101, heat shock protein 101; NPC1, Niemann–Pick C1; PfNCR1, P. falciparum Niemann-Pick Type C1-related protein; PEXEL, Plasmodium EXport ELement; PPM, parasite plasma membrane; PTEX, Plasmodium Translocon of EXported proteins; PV, parasitophorous vacuole; PVM, PV membrane. Beck JR, Ho CM. Transport mechanisms at the malaria parasite-host cell interface. PLoS Pathog. 2021 17(4):e1009394. PMID: 33793667
Other associated proteins
| PFID | Formal Annotation |
|---|---|
| PF3D7_0107500 | Niemann-Pick type c1-related protein |
| PF3D7_1116800 | heat shock protein 101 chaperone protein ClpB2 |
| PF3D7_1323500 | PEXEL protease plasmepsin V |
| PF3D7_1471100 | exported protein 2 |