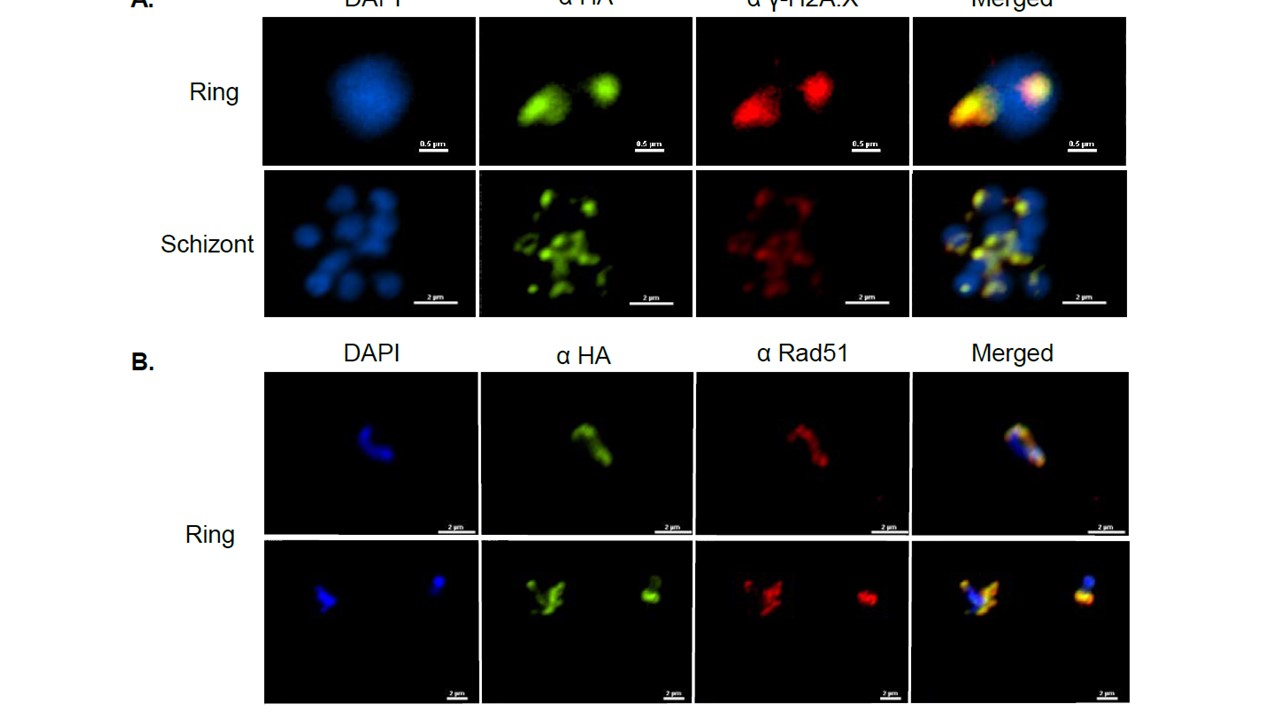
PfSR1 is recruited to the site of damaged DNA and interacts with phosphorylated H2A. (A). immunofluorescence assay demonstrating that PfSR1 is associated with γ-PfH2A in the nucleus of early (upper panel) and late stage (lower panel) PfSR1-glmS parasites 15 minutes after X-ray irradiation. (B). Immunofluorescence assay demonstrating that PfSR1 co-localize with PfRad51 in the nucleus of ring stage PfSR1-glmS parasites 15 minutes after X-ray We found a strong association (78/83 independently counted nuclei) between PfSR1 and the site of DNA damage in both early and late stages parasites (A). In addition, we found that in irradiated ring stage parasites PfSR1 co-localizes with PfRad51 (B, 39/50 independently counted nuclei), providing additional support for its recruitment to the site of DNA damage.
Goyal M, Singh BK, Simantov K, Kaufman Y, Eshar S, Ron D. An SR protein is essential for activating DNA repair in malaria parasites. J Cell Sci. 2021 jcs.258572.
Other associated proteins
| PFID | Formal Annotation |
|---|---|
| PF3D7_0617800 | histone H2A |
| PF3D7_1131100 | serpentine receptor 1, putative, PfSr1 |