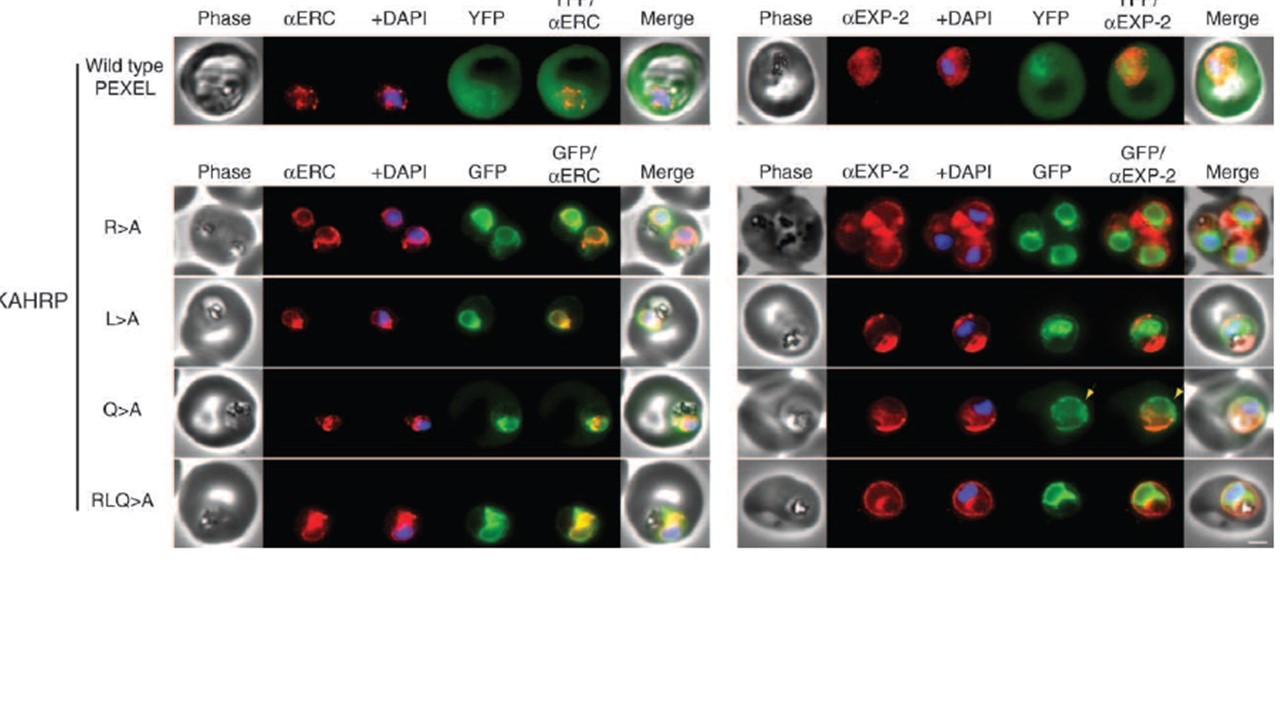
KAHRP chimaeras localise to the endoplasmic reticulum in addition to the parasitophorous vacuole or erythrocyte cytosol. Images captured by immunofluorescence microscopy show substantial colocalisation of KAHRP chimaeras with the endoplasmic reticulum protein ERC (left panels). Intraparasitic fluorescence is also evident when chimaeras were colocalised with the parasitophorous acuole membrane protein EXP-2 (right panels). Only the wild type (WT) PEXEL chimaera (uppermost panels) is efficiently exported to the erythrocyte cytosol, but low-level fluorescence in the erythrocyte cytosol was observed for the KAHRPQ>A chimaera. All mutants show
some accumulation within the parasitophorous vacuole but while the KAHRPQ>A chimaera colocalises with ERC less than the other KAHRP mutants (left panel), it accumulates more in the parasitophorous vacuole in slightly older parasites (right panel). Accumulation of the KAHRPQ>A chimaera in the parasitophorous vacuole appeared inverse to the ‘necklace of beads’ observation described previously (7; see yellow arrows in right panel). The white bar in the last panel corresponds to 2 mm for each of the panels within the figure. Anaguano D, Adewale-Fasoro O, Vick GW, Yanik S, Blauwkamp J, Fierro MA, Absalon S, Srinivasan P, Muralidharan V. Plasmodium RON11 triggers biogenesis of the merozoite rhoptry pair and is essential for erythrocyte invasion. PLoS Biol. 2024 22(9):e3002801 PMID: 39292724.
Other associated proteins
| PFID | Formal Annotation |
|---|---|
| PF3D7_0202000 | knob-associated histidine-rich protein |
| PF3D7_1471100 | exported protein 2 |