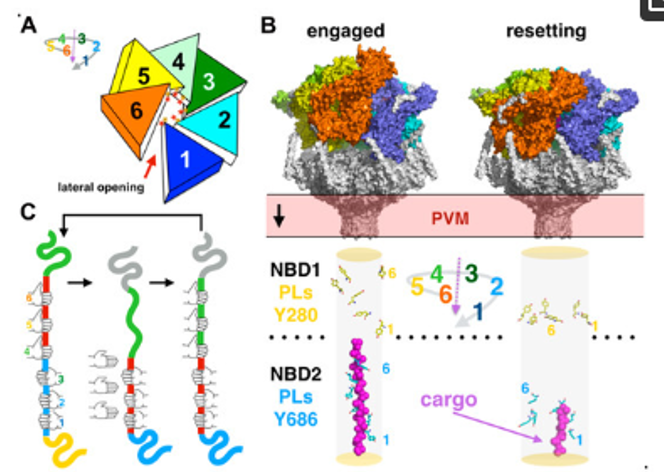
Spiral staircase hexameric assembly and substrate translocation in HSP101. (A) Schematic of the splayed spiral staircase assembly of HSP101. Each ATPase monomer is colored differently. (B) The two conformational states of PTEX resolved by cryo-EM with the conformations adopted by each of the twelve conserved pore loop tyrosines; the observed unfolded cargo is shown as magenta spheres. (C) Schematic representation of a cargo threading cycle in the NBD2 of HSP101. Each hand represents a pore loop in the NBD2. The ‘active hand’ pore loops 4–6 move up and down the ATPase translocation pore and thread it to the ‘static hand’ pore loops 1–3. Day CJ, Favuzza P, Bielfeld S, Haselhorst T, Seefeldt L, Hauser J, Shewell LK, Flueck C, Poole J, Jen FE, Schäfer A, Dangy JP, Gilberger TW, França CT, Duraisingh MT, Tamborrini M, Brancucci NMB, Grüring C, Filarsky M, Jennings MP, Pluschke G. The essential malaria protein PfCyRPA targets glycans to invade erythrocytes. Cell Rep. 2024 43(4):114012