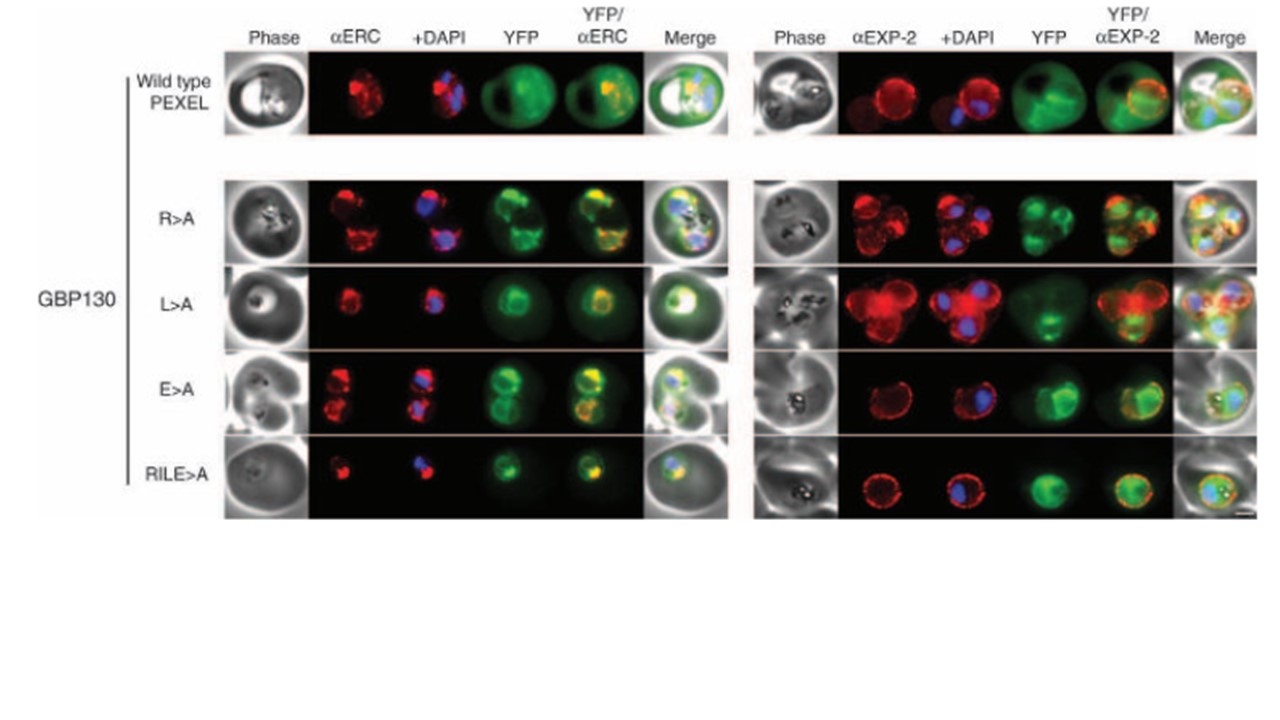
GBP130 chimaeras localise to the endoplasmic reticulum in addition to the parasitophorous vacuole or erythrocyte cytosol. Images captured by immunofluorescence microscopy show substantial colocalisation of GBP130 chimaeras with the endoplasmic reticulum protein ERC (left panels). Intraparasitic fluorescence is also evident when chimaeras were colocalised with the parasitophorous vacuole membrane protein EXP-2 (right panels). Only the wild-type PEXEL chimaera is efficiently exported to the erythrocyte cytosol (uppermost panels). All mutants show some accumulation within the parasitophorous vacuole and low-level fluorescence in the erythrocyte cytosol (lower panels), and this is evident, upon careful examination, in previously published images of these chimaeras (19). The presence of GBP130 RILE>A in the saponin supernatant in Figure 4 suggests localisation predominantly in the parasitophorous vacuole (signal sequence processed). This is confirmed by immunofluorescence microscopy (compare GBP130 RILE>A in this figure to KAHRP RLQ>A in Figure 6, the latter of which retains the signal sequence and localises more in the ER as a result). The white bar in the last panel corresponds to 2 mm for each of the panels within the figure. Boddey JA, Moritz RL, Simpson RJ, Cowman AF. Role of the Plasmodium export element in trafficking parasite proteins to the infected erythrocyte. Traffic. 2009 10(3):285-99.
Other associated proteins
| PFID | Formal Annotation |
|---|---|
| PF3D7_1016300 | glycophorin binding protein |
| PF3D7_1471100 | exported protein 2 |