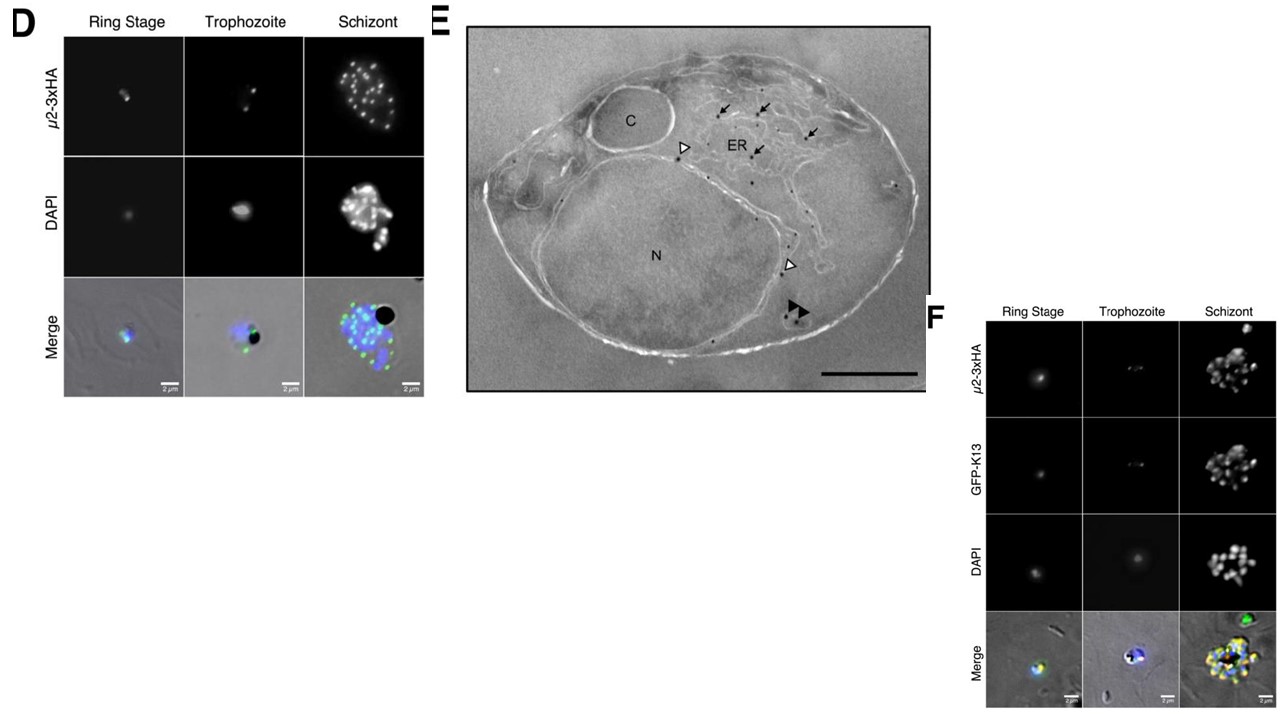
Plasmodium falciparum AP-2μ is localised to a non-canonical cytoplasmic compartment D Localisation of AP-2μ-3xHA (green) across the asexual life cycle by anti-HA IFA, counter-stained for parasite DNA with DAPI (blue). Images shown are representative of more than 100 cells examined at each stage; merge is the superimposition of each channel on a brightfield image. E Immunoelectron micrograph of a representative young intra-erythrocytic trophozoite. AP-2μ-3xHA parasites probed with an anti-HA rabbit antibody and a secondary antibody 18 nm goldIn recent studies, K13 has been localised to conspicuous membranous structures in the cytosol 36, and these superficially resemble the structures labelled by AP-2μ here (D, E). Given the apparent similarity in cellular distribution and the importance in ring-stage artemisinin susceptibility in vitro of both molecules, we hypothesised that AP-2μ and K13 localise to the same cytosolic compartment. We overexpressed an episomally-encoded N-terminal GFP-K13 fusion protein in our AP-2μ-3xHA-expressing line and observed a striking similarity in signal distribution between the K13 and AP-2μ in fixed ring and schizont stages (F).
Modification of an atypical clathrin-independent AP-2 adaptin complex of Plasmodium falciparum reduces susceptibility to artemisinin. Ryan C. Henrici, Rachel L. Edwards, Martin Zoltner, Donelly A. van Schalkwyk, Melissa N. Hart, Franziska Mohring, Robert W. Moon, Stephanie D. Nofal, Avnish Patel, Christian Flueck, David A. Baker, doi: https://doi.org/10.1101/621078