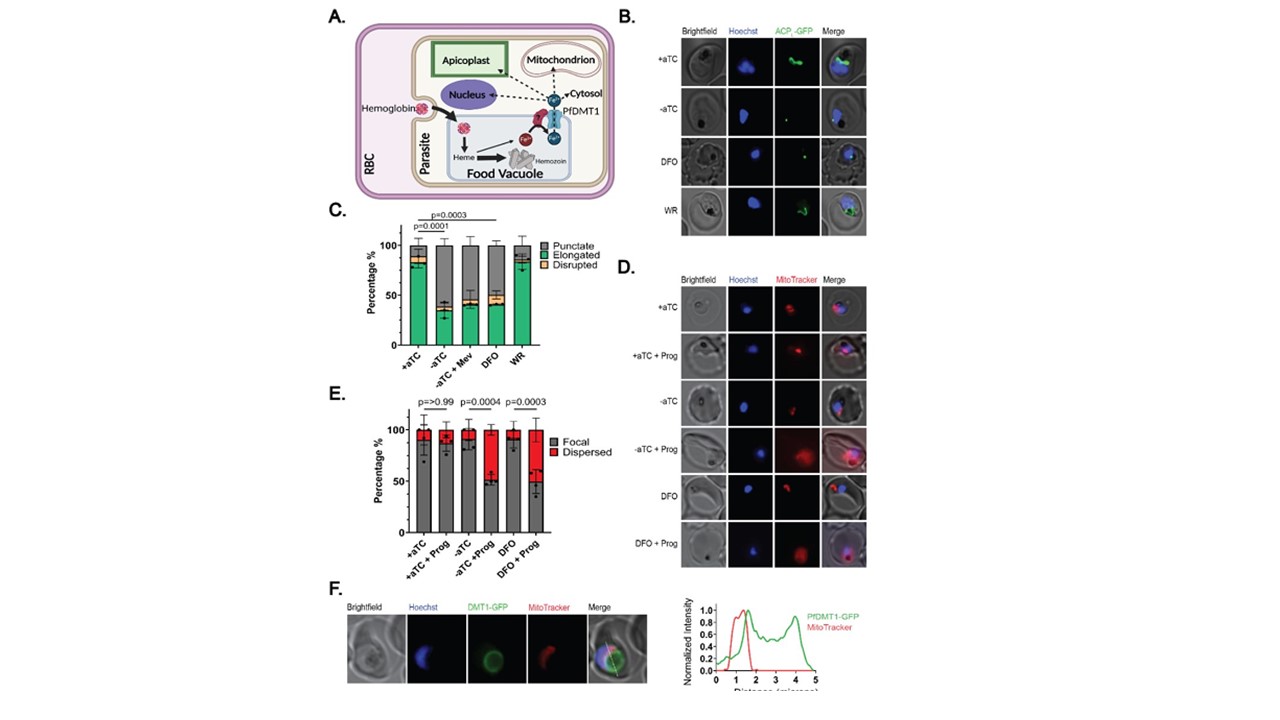
PfDMT1 KD impairs apicoplast and mitochondrial functions. (A) Proposed model of iron export via PfDMT1 to support broad cellular metabolism (figure created using BioRender.com). (B) Live-cell images of PfDMT1 KD parasites treated ± aTC, 60 µM DFO, 50 µM DL-mevalonate (Mev) or 5 nM WR. The apicoplast was visualized by ACPL-GFP expressed in PfMev NF54 parasites. (C) Population analysis of apicoplast morphology in each experimental condition. (D) Live-cell images of PfDMT1 KD parasites stained with MitoTracker™ Red CMXRos after culture ± aTC or treatment with 60 µM DFO ±5 µM proguanil (added 12 hours before imaging). (E) Population analysis of mitochondrial polarization in each experimental condition. For (C) and (E) each data point is the mean of biological replicates where 12-20 parasites were imaged and scored. A two-tailed unpaired t-test was utilized to calculate the given p-values (full comparison given in Table S1 and S2). In all experiments, parasites were synchronized to a 4-hour window and treated at t=0 hours post-synchronization to rings. (F) Live-cell image of PfDMT1-GFP and MitoTracker™ Red CMXRos signals. The intensity plot depicts the normalized GFP and MitoTracker fluorescence as a function of distance along the white line. Loveridge KM, Sigala PA. Identification of a divalent metal transporter required for cellular iron metabolism in malaria parasites. bioRxiv [Preprint]. 2024 2024.05.10.587216. PMID: 38798484;
€€€€€