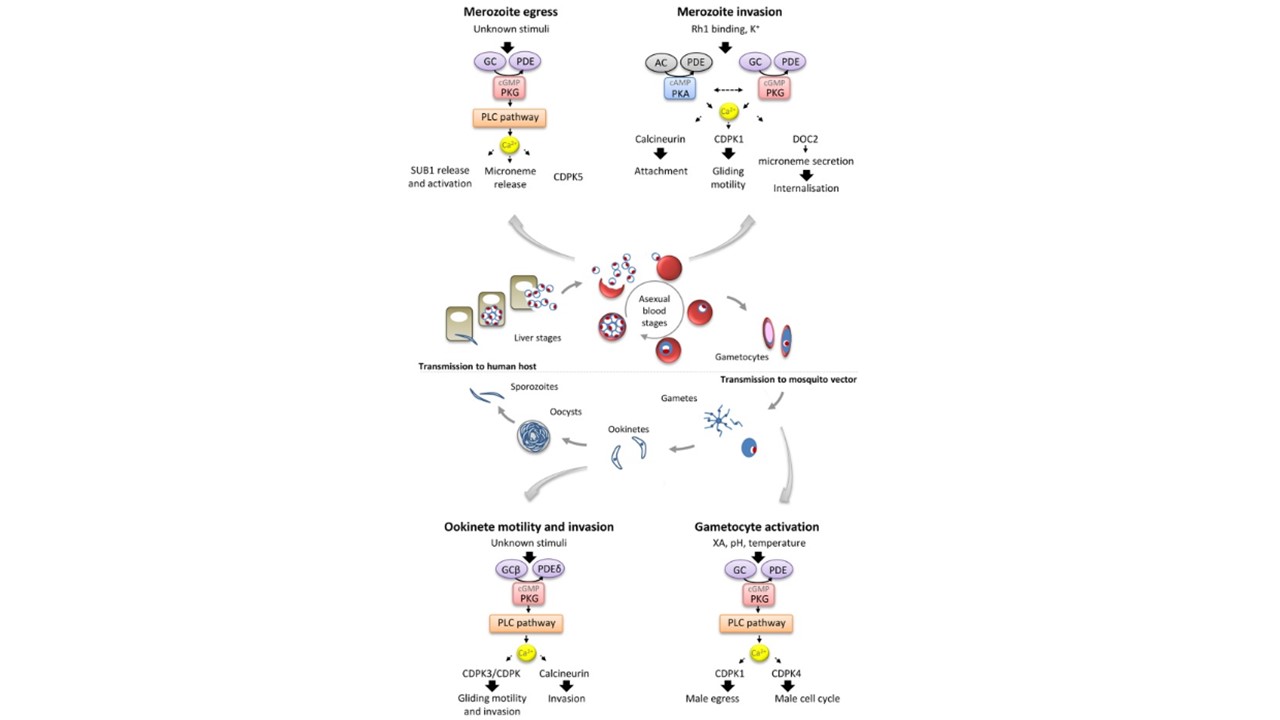
Conserved interplay between Ca2+, cyclic nucleotides and the PI-PLC pathway at different stages of the Plasmodium life cycle and downstream stage-specific effectors. During merozoite egress, activation of PKG by high cGMP levels triggers hydrolysis of PIP2 and Ca2+ mobilisation from internal stores. This Ca2+ signal is required for the rupture of the PVM and for egress from the erythrocyte possibly through the activation of SUB1 and CDPK5. Prior to the invasion of erythrocyte by merozoites, different stimuli, including contact with the host cell, stimulate a cAMP-dependent Ca2+ mobilisation. Activity of PKG is also required for merozoite invasion and dynamic phosphorylation of CDPK1 which could regulate the acto-myosin motor. Downstream of Ca2+, attachment of the parasite to the host cell involves calcineurin activation while DOC2-dependent secretion of EBA175 is required for further internalisation of the parasite. During gametocyte activation, external stimuli trigger the elevation of cGMP levels by regulating unknown PDE and GC to activate PKG. This leads to the activation of the PI-PLC pathway to produce IP3 and the mobilisation Ca2+ from internal stores. Ca2+ further activates CDPK4 and CDPK1 to control cell cycle events and egress respectively. In motile ookinetes, cGMP levels are tightly regulated by GCβ and PDEδ. Activity of PKG is required to maintain high PIP, PIP2 and Ca2+ levels. Altered Ca2+ signalling through CDPK3 and another unidentified CDPK leads to a reduction in gliding speed while calcineurin is only essential for invasion of the mosquito midgut wall. Dotted arrows linking Ca2+ and effector proteins denote the fact that direct links between the cyclic nucleotide-dependent Ca2+ signal and the activation of the effectors were not experimentally demonstrated. Brochet M, Billker O. Calcium signalling in malaria parasites. Mol Microbiol. 2016 100(3):397-408.
PubMed Article: Calcium signalling in malaria parasites