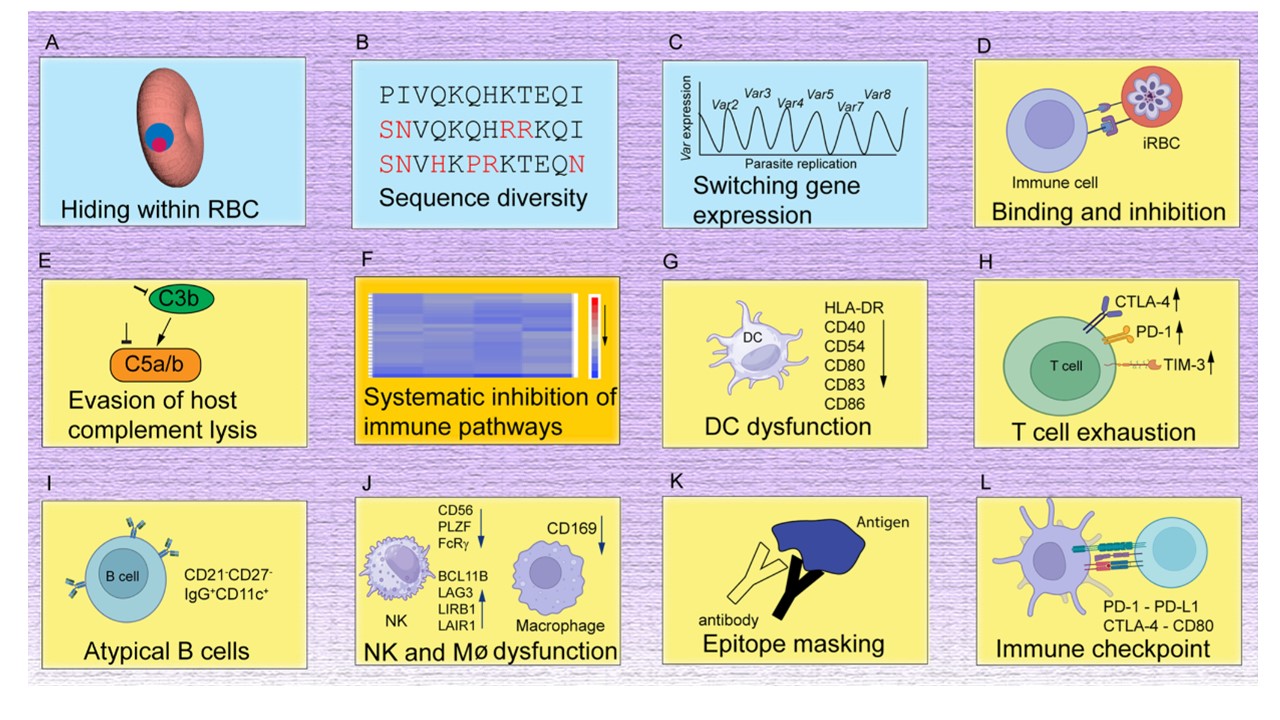
Major mechanisms of immune evasion in malaria. (A) Parasite lives within RBCs, avoiding various intracellular killing mechanisms, although parasite proteins expressed on the surface of RBCs can be recognized by the host immune system. (B) Parasite molecules exposed to host immunity are highly polymorphic, allowing survival under strain-specific immunity. (C) Expression of a different copy of a variant antigen gene (var) can also evade immunity against a previously expressed variant. (D) Binding of parasite proteins such as PfEMP1 and PfRIFIN to various host cells can induce immune inhibition and avoid clearance by the spleen. (E) Binding of parasite proteins to molecules in the host complement system blocks complement-mediated lysis and killing of the parasites. (F) Genome-wide transcriptional analyses show down-regulation of host genes in many immune pathways, including B and T cell activations. (G) DC dysfunction with reduced expression of many surface proteins required for T cell activation. (H) Malaria parasite infection also leads to T cell dysfunction and exhaustion with increased expression of T cell exhaustion and senescence markers. (I) Expansion of a group of B cells with reduced B cell receptor signaling was recognized in malaria. The status and functions of the atypical B cell required further investigation. (J) Dysfunction of macrophages and NK cells was also observed in malaria. Dysfunction of erythropoietic island macrophage can also cause anemia. (K) Antibodies from prior exposure or immunization may block the binding of new antibodies generated. (L) Many immune checkpoint pathways are activated in malaria, and blockade of the pathways can reverse immune inhibition, inhibit parasite growth, and enhance host survival in rodent malaria models. Note that many of these elements are overlapping and can be integrated into a broad systematic immune inhibition. Su XZ, Xu F, Stadler RV, Teklemichael AA, Wu J. Malaria: Factors affecting disease severity, immune evasion mechanisms, and reversal of immune inhibition to enhance vaccine efficacy. PLoS Pathog. 2025 PMID: 39847577