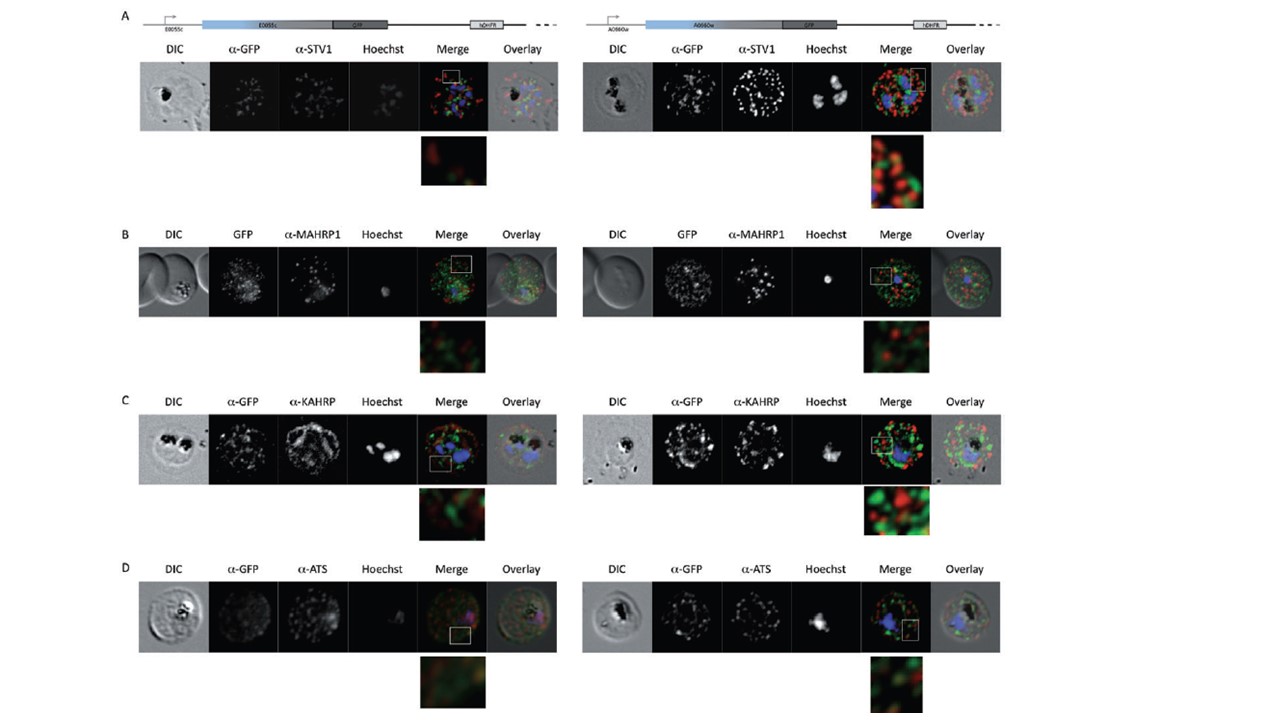
Co-immunofluorescence microscopy of GFP transfectant lines. Co-immunofluorescence analysis on erythrocytes infected with PFE55INT (left side) or PFA660INT (right side), using anti-sera directed against STEVOR (A), MAHRP1 (B), KAHRP (C), PfEMP1 (D). Fluorescence channels are shown individually in black/white for highest contrast. All images are maximal projections of Z-stack serial sections. In merge image green, GFP; red, STEVOR (A), MAHRP (B), KAHRP (C) or ATS domain of PfEMP1 (D); blue, Hoechst. Inset shows enlargement of merge (white box). Partial colocalization of GFP signals can be observed only upon staining of PfEMP1. In neither erythrocytes infected with PFE55INT nor PFA660INT could colocalization between the GFP signal and either STEVOR, MAHRP1 or KAHRP be observed (C). We did note a slight colocalization between both PFE55/PFA660–GFP and PfEMP1; however, the bulk of the GFP signal was distinct from the PfEMP1 staining (D).
Külzer S, Rug M, Brinkmann K, Cannon P, Cowman A, Lingelbach K, Blatch GL, Maier AG, Przyborski JM. Parasite-encoded Hsp40 proteins define novel mobile structures in the cytosol of the P. falciparum-infected erythrocyte. Cell Microbiol. 2010 12(10):1398-420.
Other associated proteins
| PFID | Formal Annotation |
|---|---|
| PF3D7_0113700 | heat shock protein 40, type II |
| PF3D7_0202000 | knob-associated histidine-rich protein |
| PF3D7_0501100 | co-chaperone j domain protein jdp |
| PF3D7_1370300 | membrane associated histidine-rich protein |
| PfEMP1 | PfEMP1 |