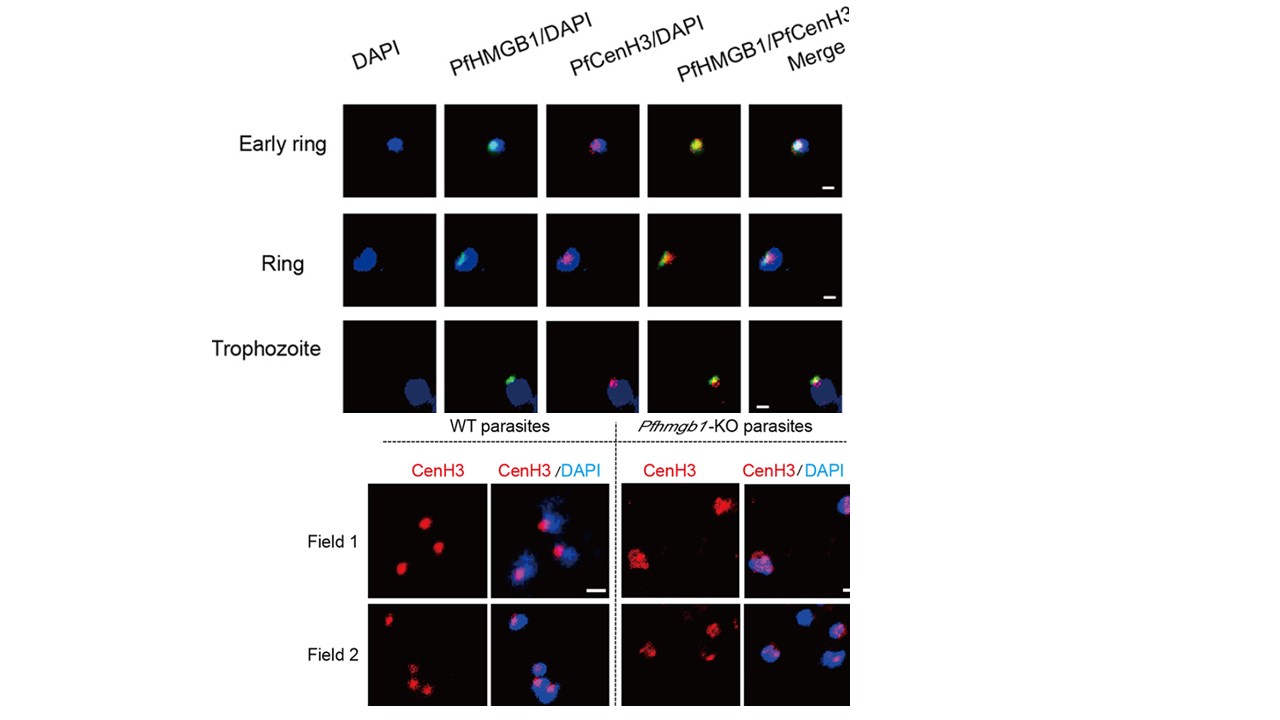
PfHMGB1 interacts with perinuclear centromeres. Upper panel: Co-IFA assay of PfHMGB1-HATy1 (mouse anti-Ty1 antibody, green) and GFP-PfCenH3 (rabbit anti-GFP antibody, red) in the ring or trophozoite-stage parasites of PfHMGB1-HA-Ty1::GFP-PfCenH3 line. Nuclear DNA was stained by DAPI (blue). Bars, 1mm. Lower panel: IFA assay of GFP-PfCenH3 with anti-GFP antibody in the ring-stage parasites of Pfhmgb1-KO and WT control. Nuclear DNA was stained by DAPI (blue). Bars, 1mm. Co-IFA assays confirmed the colocalization of PfHMGB1 and the centromere superdomain (upper panel). They mainly formed a single focus at a unique site on the nuclear periphery, which is consistent with a previous description of centromere distribution in the nucleus of P. falciparum (35, 36) and in fission yeast. We observed that, to some extent, the size of a single centromeric focus was larger in the nucleus of Pfhmgb1-KO parasites (lower panel), supportive of a reduced interactome within the centromere superdomain which was previously described by 3D genome modeling.
Lu B, Liu M, Gu L, Li Y, Shen S, Guo G, Wang F, He X, Zhao Y, Shang X, Wang L, Yang G, Zhu Q, Cao J, Jiang C, Culleton R, Wei G, Zhang Q. The Architectural Factor HMGB1 Is Involved in Genome Organization in the Human Malaria Parasite Plasmodium falciparum. mBio. 2021 12(2):e00148-21. PMID: 33906919.
Other associated proteins
| PFID | Formal Annotation |
|---|---|
| PF3D7_1027700 | centrin-3 |