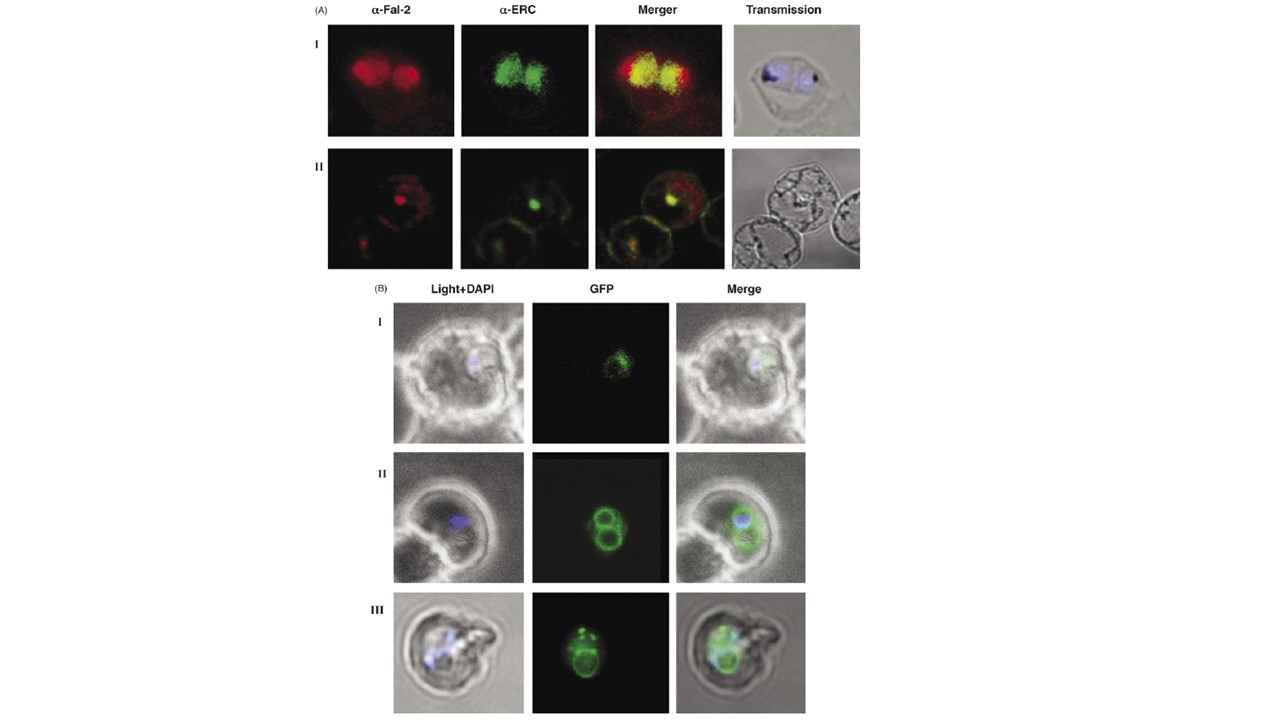
Trafficking of falcipain 2 occurs via ER-Golgi network and is sensitive to brefeldin A treatment. (A) Confocal microscopic images of 3D7 parasites with (panel II) and without (panel I) brefeldin A treatment. The first image in each set represents the falcipain-2 staining, the second is the staining of PfERC, with an overlay of these images in the third panel. The fourth image is in bright field. Parasite nuclei were stained with DAPI (blue). Panel I, a trophozoite stage parasite showing co-localization of falcipain-2 and PfERC without brefeldin A treatment. Panel II, a confocal microscope image of 3D7 parasites showing immuno-localization of falcipain-2 protein in brefeldin A treated parasites. (B) Confocal microscope images of Fal2-S1 parasites treated with brefeldin A (panel I). The GFP fluorescence was observed in a contracted compartment in brefeldin A treated parasites as compared to control parasite (panel II) where GFP fluorescence was present around nucleus, in PV and in the food vacuole membrane. Redistribution of GFP was observed after the release of BFA block (panel III). The first image (left to right) in each set represents the bright field image, the second is the fluorescence signal from the GFP chimeric protein and an overlay of these two images is shown in the third image. Falcipain-2 localized in the food vacuole and also in the endoplasmic reticulum as evident from its co-localization with the endoplasmic reticulum resident protein (PfERC) (panel I), thereby suggesting the translocation of falcipain-2 via ER/Golgi network. We also observed presence of falcipain-2 in PVM and compartments other than food vacuole at the segmenter stage. As shown in Fig. A, panel II and B, panel I, trafficking of falcipain-2 as well as the fusion proteins was sensitive to brefeldin A treatment; this trafficking was not affected by carrier solvent, ethanol, which was added to the control Fal2-S1 line (B, panel II). In Fal2-S1 parasites and 3D7 parasites, BFA treatment resulted in accumulation of falcipain-2 and GFP in a contracted compartment close to the nucleus (B, panel I). Co-localization of falcipain-2 with PfERC in 3D7 line provided evidence that this contracted compartment represents an ER (A, panel II). Removal of brefeldin A resulted in normal growth and distribution of GFP labeling in the treated parasite (B, panel III). These results suggested that trafficking of falcipain-2 occurs by classical secretory route that involves transport of protein from ER to the Golgi-like structure in a brefeldin A sensitive manner.
Dasaradhi PV, Mohmmed A, Kumar A, Hossain MJ, Bhatnagar RK, Chauhan VS, Malhotra P. A role of falcipain-2, principal cysteine proteases of Plasmodium falciparum in merozoite egression. Biochem Biophys Res Commun. 2005 336(4):1062-8
Other associated proteins
| PFID | Formal Annotation |
|---|---|
| PF3D7_1108600 | endoplasmic reticulum-resident calcium binding protein |
| PF3D7_1115300 | falcipain 2' cysteine proteinase falcipain 2b |