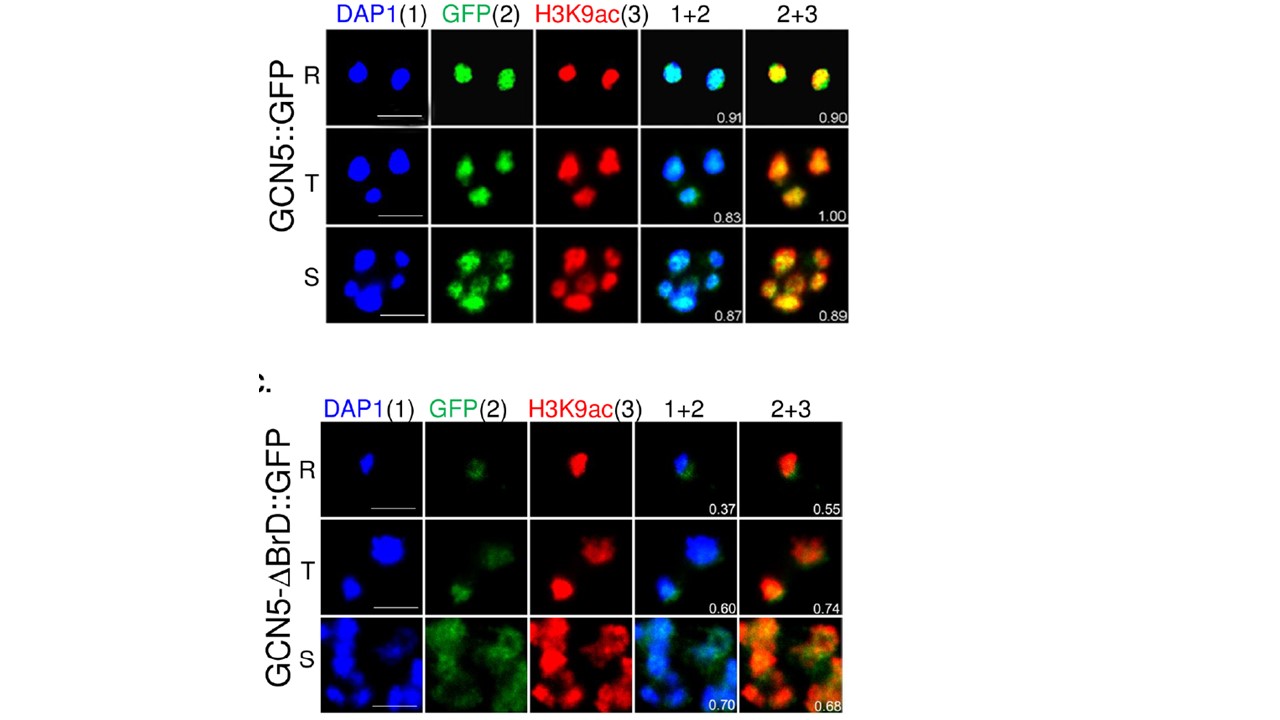
Domain deletions affect the abundance and localization of active histone marks and the integrity of the PfGCN5 complex. Co-localization of full-length PfGCN5 (GCN5::GFP) (upper panel) or truncated PfGCN5 (GCN5-ΔBrd::GFP) (lower panel) with H3K9ac and DAPI by IFA with anti-GFP and H3K9Ac antibodies. Note the expansion of the truncated PfGCN5-ΔBrD::GFP and H3K9ac beyond the periphery of the euchromatin areas demarcated by DAPI staining. In the PfGCN5::GFP parasites, there were high levels of colocalizations between the DAPI and PfGCN5 (r2 =0.83–0.91), and between PfGCN5 and H3K9ac (r2 = 0.89–1.0), indicating that PfGCN5 is tightly associated with H3K9ac in the active euchromatin area demarcated by DAPI staining (upper panel). However, the levels of the colocalization substantially decreased in the PfGCN5-ΔBrD::GFP parasites (r2 = 0.37–0.74) (lower panel). Some signals of the truncated PfGCN5 were localized beyond the DAPI area, suggesting that PfGCN5 might have spread to the perinuclear heterochromatic area.
Miao J, Wang C, Lucky AB, Liang X, Min H, Adapa SR, Jiang R, Kim K, Cui L. A unique GCN5 histone acetyltransferase complex controls erythrocyte invasion and virulence in the malaria parasite Plasmodium falciparum. PLoS Pathog. 2021 17(8):e1009351.