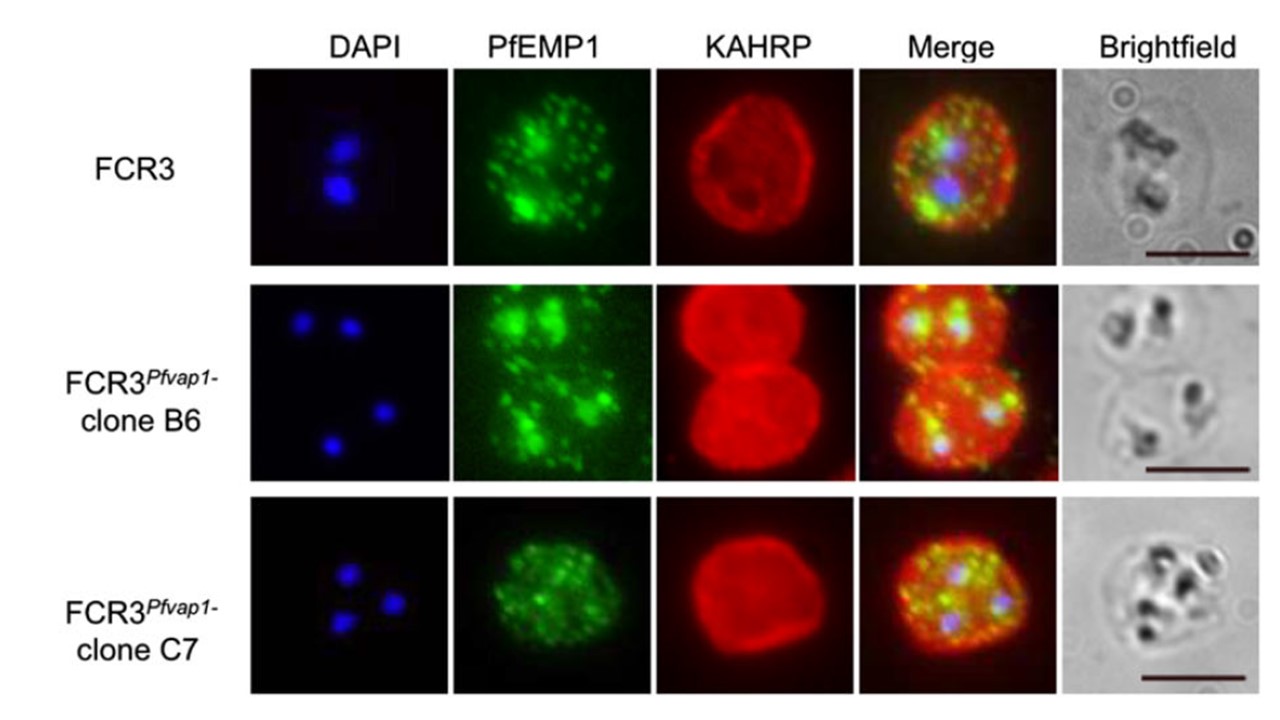
FCR3Pfvap1Δ knockout parasites show a reduction in cytoadhesion. parasites export PfEMP1 and KAHRP in a manner comparable with wild-type FCR3 parasites, suggesting that there is no impairment or retention of these proteins in mutant parasites. Scale bar = 5 μm. To ascertain that the observed decrease in binding levels in FCR3Pfvap1Δ were not due to off-target events, such as a concomitant deletion of KAHRP as has been reported for the complete rex1 knockouts in 3D7, we verified targeting of key proteins to different cellular compartments. Immunolabelling of FCR3Pfvap1Δ indicated that these parasites have KAHRP at the IE membrane and trafficking of PfEMP1 was comparable with FCR3
Nacer A, Claes A, Roberts A, Scheidig-Benatar C, Sakamoto H, Ghorbal M, Lopez-Rubio JJ, Mattei D. Discovery of a novel and conserved Plasmodium falciparum exported protein that is important for adhesion of PfEMP1 at the surface of infected erythrocytes. Cell Microbiol. 2015 17(8):1205-16.
Other associated proteins
| PFID | Formal Annotation |
|---|---|
| PF3D7_0202000 | knob-associated histidine-rich protein |
| PF3D7_0936500 | virulence-associated protein 1 |