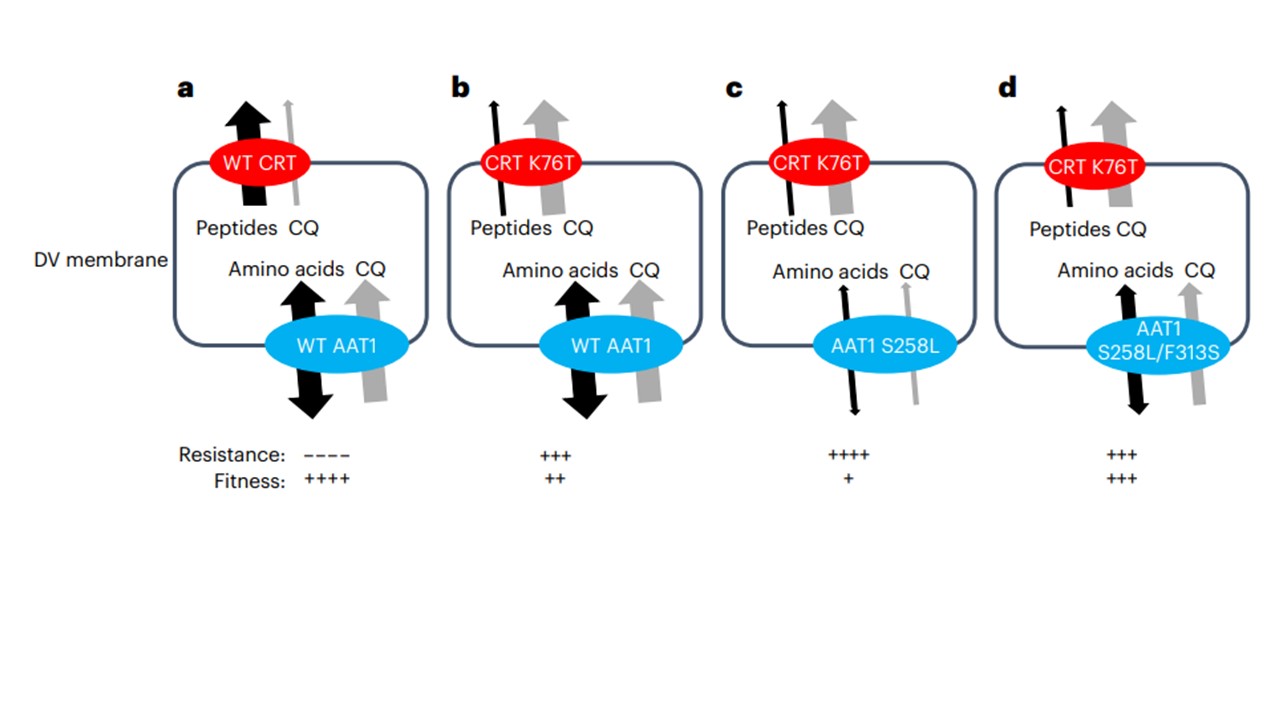
Model for involvement of pfaat1 haplotypes in CQ resistance and fitness. pfCRT (red) and pfAAT1 (blue) are both situated in the digestive vacuole (DV) membrane. a, WT pfCRT and pfAAT1 transport peptides and aromatic amino acids, respectively, as well as CQ. b, pfCRT K76T exports CQ from the DV away from its site of action, leading to elevated resistance but transports peptides inefficiently leading to a loss of fitness. c, pfAAT1 S258L reduces entry of CQ into the DV, leading to elevated resistance, but amino acid flux is affected, leading to a loss of fitness. d, The pfAAT1 S258L/F313S double mutation increases CQ influx in comparison with the S258L alone but the amino acid transport function is restored, leading to reduced IC50 values and increased fitness in the absence of drug treatment.
Amambua-Ngwa A, Button-Simons KA, Li X, Kumar S, Brenneman KV, Ferrari M, Checkley LA, Haile MT, Shoue DA, McDew-White M, Tindall SM, Reyes A, Delgado E, Dalhoff H, Larbalestier JK, Amato R, Pearson RD, Taylor AB, Nosten FH, D'Alessandro U, Kwiatkowski D, Cheeseman IH, Kappe SHI, Avery SV, Conway DJ, Vaughan AM, Ferdig MT, Anderson TJC. Chloroquine resistance evolution in Plasmodium falciparum is mediated by the putative amino acid transporter AAT1. Nat Microbiol. 2023
Other associated proteins
| PFID | Formal Annotation |
|---|---|
| PF3D7_0629500 | amino acid transporter aat1 |