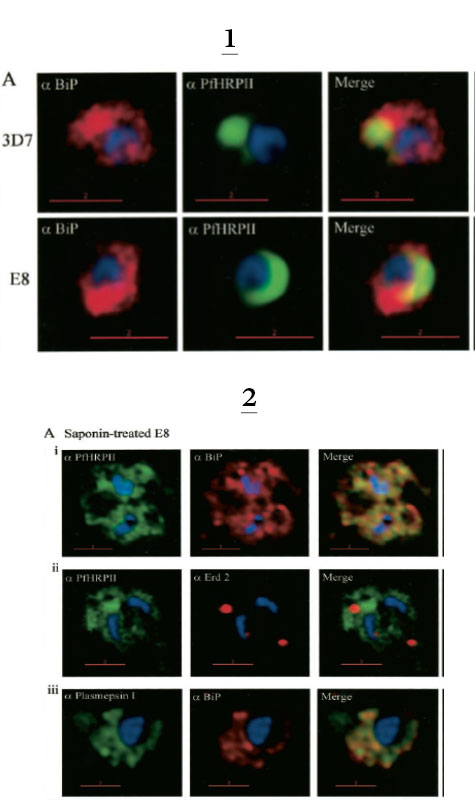
1. Comparative analysis of secretory recruitment of HRPII, export of parasite proteins in E8- and 3D7-infected erythrocytes, and distribution of total PfHRPII, PfHRPIImyc, plasmepsin I, and BiP in parasites and isolate food vacuoles. A, ring-infected red cells from E8 and 3D7-strains were treated with brefeldin A, stained for PfBiP (red), PfHRPII (green), and Hoechst dye (blue) in indirect immunofluorescence assays, and imaged using DeltaVision. Single optical sections showing a merge of red and green as yellow are shown. In the presence of BFA, all cell-associated HRPIIs detected in both E8 and 3D7 reside within regions that stain with the ER marker PfBiP, confirming that HRPII is recruited to the secretory pathway.
2. Membrane association of parasite-HRPII in E8 and ACPGFP cells. A, 30–33-h trophozoite-infected red cells from 3D7 and E8 strains were attached to poly-L-lysine-coated cover slips and then permeabilized with 0.01% saponin to release the erythrocytic contents. The adherent cells were then fixed with formaldehyde and probed for the distribution of the indicated proteins by indirect immunofluorescence microscopy. HRPII and plasmepsin I show substantial colocalization with PfBiP in E8 cells but not markers of the Golgi (ERD2).
Akompong T, Kadekoppala M, Harrison T, Oksman A, Goldberg DE, Fujioka H, Samuel BU, Sullivan D, Haldar K. Trans expression of a Plasmodium falciparum histidine-rich protein II (HRPII) reveals sorting of soluble proteins in the periphery of the host erythrocyte and disrupts transport to the malarial food vacuole. J Biol Chem. 2002 277:28923-33.
Other associated proteins
| PFID | Formal Annotation |
|---|---|
| PF3D7_0831800 | histidine-rich protein II |
| PF3D7_0917900 | PfHsp70-2 |
| PF3D7_1407900 | plasmepsin I |