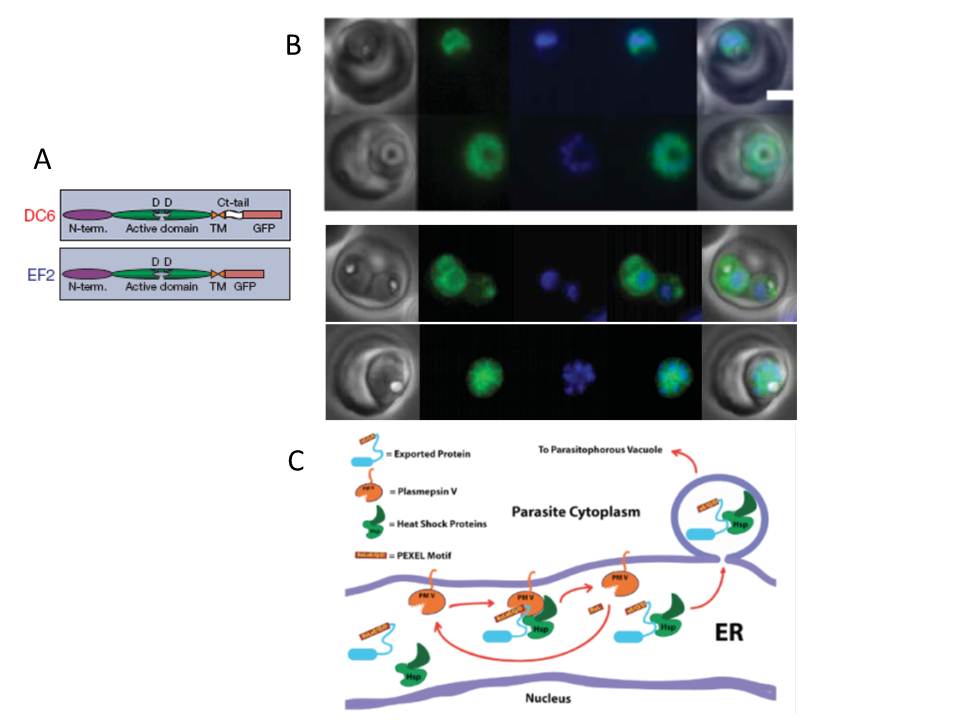
A. Schematic of PMV and C-terminal integrants. SP, signal peptide; N-term., N-terminal domain; Ct, C-terminal; TM, transmembrane region.
B. Proteins destined for export are synthesized in the endoplasmic reticulum (ER) and cleaved at a conserved (PEXEL) motif, which allows translocation into the host cell via an ATP-driven translocon called the PTEX complex. We report that plasmepsin V, an ER aspartic protease recognizes the PEXEL motif and cleaves it at the correct site. Live fluorescence images of DC6 and EF2. Left to right: phase, GFP, DAPI, fluorescence merge and total merge. Scale bar, 2 mm.
C. Schematic model of exported protein interactions. A PEXEL-containing protein is recognized by PMV in association with chaperones such as HSP 70 and HSP 101. PMV cleaves the PEXEL, releasing mature protein into the stewardship of the chaperones, which usher the protein through the secretory system to the translocon for export into the host erythrocyte.
Russo I, Babbitt S, Muralidharan V, Butler T, Oksman A, Goldberg DE. Plasmepsin V licenses Plasmodium proteins for export into the host erythrocyte. Nature. 2010 463(7281):632-6.