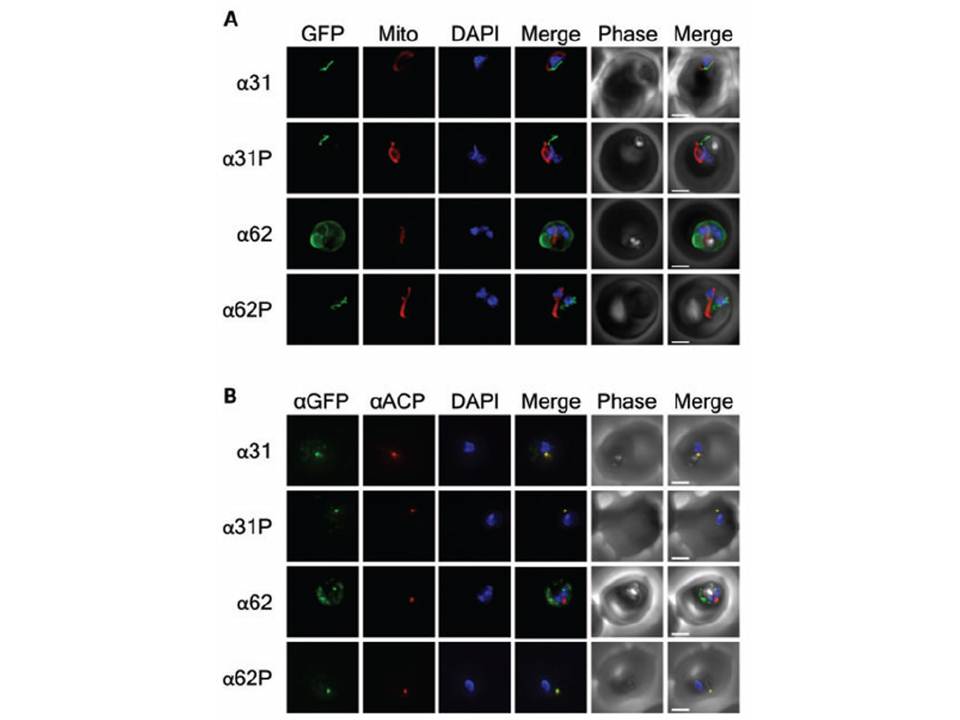
Subcellular location, processing and genomic integration of helix stabilization. Constructs Epifluorescent images of live P. falciparum erythrocytic-stage parasites expressing GFP fused to helix stabilization mutants of the ACP leader peptide. The parasites were stained with mitotracker to identify mitochondria and DAPI to identify nuclei. Image z-stacks were deconvoluted and then presented as a single combined image. Scale bar = 2 μm. B) Colocalization of GFP constructs with endogenous ACP. An antibody specific for GFP was found to colocalize with aACP antibodies, demonstrating apicoplast localization for all constructs except for a62. DAPI stains the nuclei. Scale bar = 2 μm. Any observed changes in localization are due to the stabilized helical structure, a single proline mutation was introduced into each of the engineered TPs at residue 30, producing the α31P and a62P constructs. The L30P mutation reduces the predicted average helical content for the a 31P and a62P constructs to 3% and 8%, respectively. GFP fluorescence of the a62P constructs was observed around the periphery of the parasite, consistent with accumulation in the parasitophorous vacuole, as observed for the ΔT construct. the helix-stabilizing mutations in a62 prevented this construct from localizing to the apicoplast.
Gallagher JR, Matthews KA, Prigge ST. Plasmodium falciparum apicoplast transit peptides are unstructured in vitro and during apicoplast import. Traffic. 2011 12(9):1124-38.