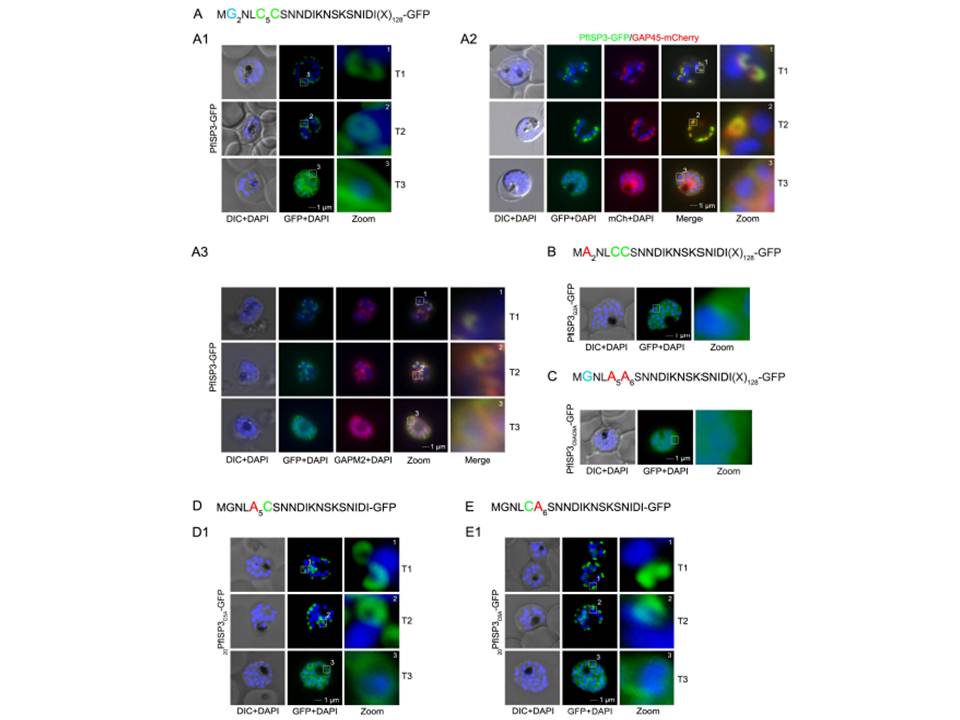
The role of N-terminal acylation for IMC membrane association of PfISP3. A. Over-expression of PfISP3-GFP showing characteristic IMC dynamics during schizogony (T1-T3) in unfixed parasites. Nuclei are stained with DAPI (blue). Enlargement of selected areas are marked with a white square and referred to as zoom. Scale bar, 1 μm (A1). A2. Co-localization of PfISP3-GFP (green) and GAP45mCherry (red) in unfixed parasites revealed their identical dynamic during schizogony. A3. Colocalization with the IMC marker GAPM2 (α-GAPM2, red) in fixed cells. Putative myristoylation and palmitoylation sites are highlighted in light blue (G2) or green (C5C6). B-C. IMC membrane association depends on the presence of N-terminal myristoylation and palmitoylation motifs. N-terminal myristoylation (B, PfISP3G2A-GFP) and palmitoylation motif mutants (C, PfISP3C5AC6A-GFP) were expressed in P. falciparum and localized in unfixed parasites. Mutation of either the myristoylation or the palmitoylation motifs resulted in a cytosolic distribution of the GFP-fusion protein (B1, C1). D-E. Mutation of the individual palmitoylation sites C5 (PfISP3C5A-GFP) or C6 (PfISP3C6A-GFP) does not interfere with IMC recruitment of the fusion protein.
Wetzel J, Herrmann S, Swapna LS, Prusty D, Peter AT, Kono M, Saini S, Nellimarla S, Wong TW, Wilcke L, Ramsay O, Cabrera A, Biller L, Heincke D, Mossman K, Spielmann T, Ungermann C, Parkinson J, Gilberger TW. The role of palmitoylation for protein recruitment to the inner membrane complex of the malaria parasite. J Biol Chem. 2014 Nov 25.
Other associated proteins
| PFID | Formal Annotation |
|---|---|
| PF3D7_1460600 | inner membrane complex sub-compartment protein 3 |