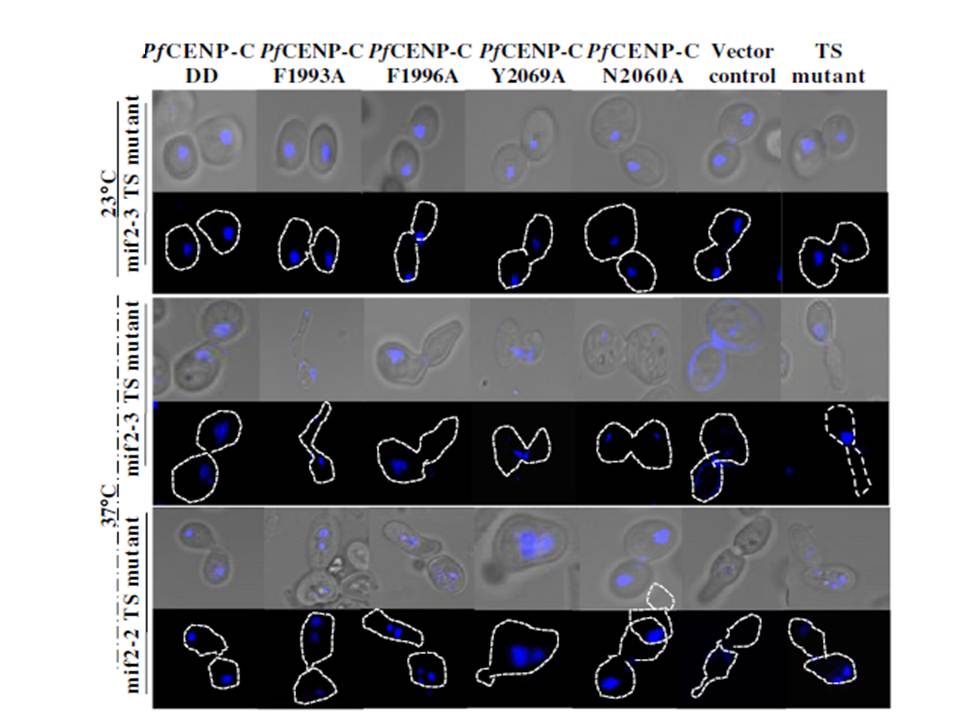
Functionally essential residues lie within the PfCENP-C dimerization domain. the phenotypic changes exhibited by the dimerization domain mutants PfCENP-CF1993A, PfCENPCF1996A, PfCENP-CY2069A and PfCENP-CN2060A were assessed at 23 and 37°C, respectively. At 23°C, mif2-3 and mif2-2 TS mutant cells expressing different PfCENP-C dimerization domains show proper chromosome segregation and normal bud morphology. At 37°C, ~90-94% of mif2-3 and mif2-2 TS mutant cells expressing PfCENP-CF1993A, PfCENP-CF1996A and PfCENP-CY2069A are mitotically arrested and unviable. This is evident by the aberrant bud morphologies with fragmented nucleus or loss of nuclear staining. These abnormal phenotypes are attributable to the failure of PfCENP-C dimerization domain mutants to dimerize resulting in impaired growth, defective chromosome segregation and loss of viability.
Verma G, Surolia N. The dimerization domain of PfCENP-C is required for its functions as a centromere protein in human malaria parasite Plasmodium falciparum. Malar J. 2014 Dec 4;13(1):475.