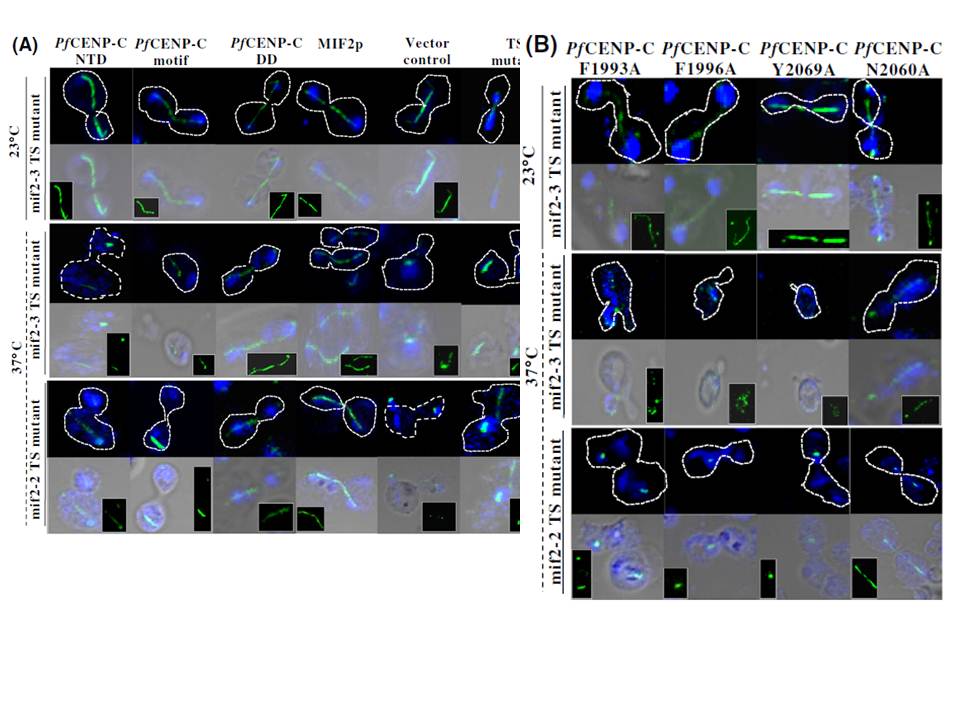
The mitotic spindle integrity is maintained by the PfCENP-C dimerization domain. (A) Confocal images showing the mitotic spindle (green) and the Hoechst stained nucleus (blue) of the mif2-3 and mif2-2 TS mutant cells expressing the PfCENP-C-NTD, -motif and -DD, region, MIF2p and empty vector alone at 23 and 37°C, respectively. At 23°C, all the mif2-3 TS mutants expressing different constructs show normal spindle structure connecting the nuclear masses across the bud and mother cell. At 37°C, both in mif2-3 and mif2-2 TS mutants except the PfCENP-C-DD and MIF2p, all the other constructs show abnormal and discontinuous spindles. The spindle morphology (green) is also shown in the insets. The broken spindle in themif2-3 and mif2-2 TS mutant cells are shown by red arrows in the insets.
B) The confocal images representing the disintegrated mitotic spindle morphologies in mif2-3 and mif2-2 TS mutant cells expressing the PfCENP-C-DD mutants, PfCENP-CF1993A, PfCENP-CF1996A and PfCENP-CY2069A while PfCENP-CN2060A show normal mitotic spindles at 37°C. NTD- N-terminal region; DD-dimerization domain.
Verma G, Surolia N. The dimerization domain of PfCENP-C is required for its functions as a centromere protein in human malaria parasite Plasmodium falciparum. Malar J. 2014 Dec 4;13(1):475.