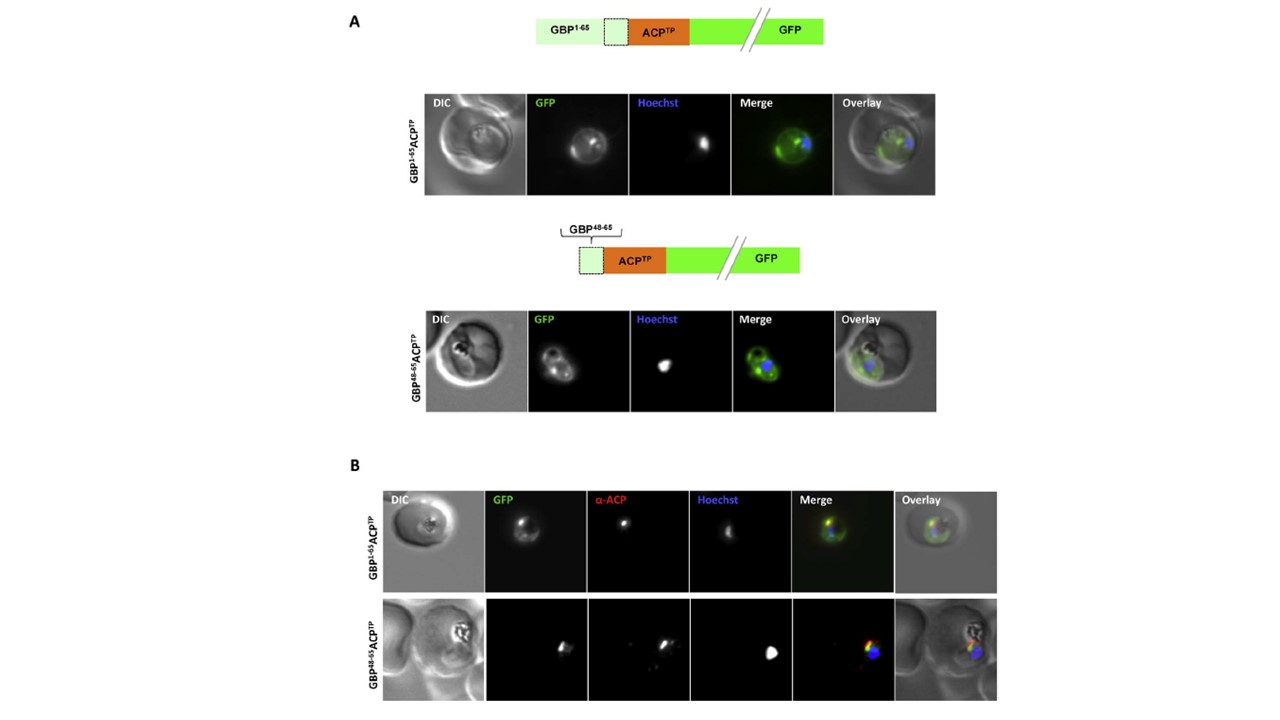
Both extended and shortened GBP130 signal peptides can replace the canonical signal peptide of an apicoplast protein. (A) Live cell imaging (B) Immunofluorescence. A schematic of each construct is shown above the respective images. DIC, differential interference contrast; GFP, green fluorescent
protein; Hoechst, nuclear staining. In merge and overlay: green, GFP; blue, Hoechst; red, anti-ACP. Traffic to the apicoplast is directed by an Nterminal canonical signal peptide which mediates entry into the secretory pathway, followed by a so-called transit peptide which is required for transport across the remaining apicoplast membranes. We wished to understand whether recessed signal peptides may target a sub-domain of the ER responsible for export to the host cell, or whether all proteins enter the secretory system at the same point. We thus generated parasites expressing the first 65aa of GBP130 fused to a transit peptide derived from acyl carrier protein (ACP). Additionally, we also fused the transit peptide to the C- domain of GBP130 (aa48-65). In both cases we could observe a mixed fluorescent phenotype, with a small bright “dot” within the parasite, and additionally a circular rim of fluorescence
surrounding the parasite (A, upper and lower panels). Analysis
using antisera against the apicoplast marker ACP verified the dotted fluorescence as the apicoplast. The fluorescence surrounding the parasite probably represents the lumen of the parasitophorous vacuole, the default target of secretory proteins lacking any further targeting signals.
Meyer C, Barniol L, Hiss JA, Przyborski JM. The N-terminal extension of the P. falciparum GBP130 signal peptide is irrelevant for signal sequence function. Int J Med Microbiol. 2017 S1438-4221(17)30195-9.
Other associated proteins
| PFID | Formal Annotation |
|---|---|
| PF3D7_0208500 | acyl carrier protein |