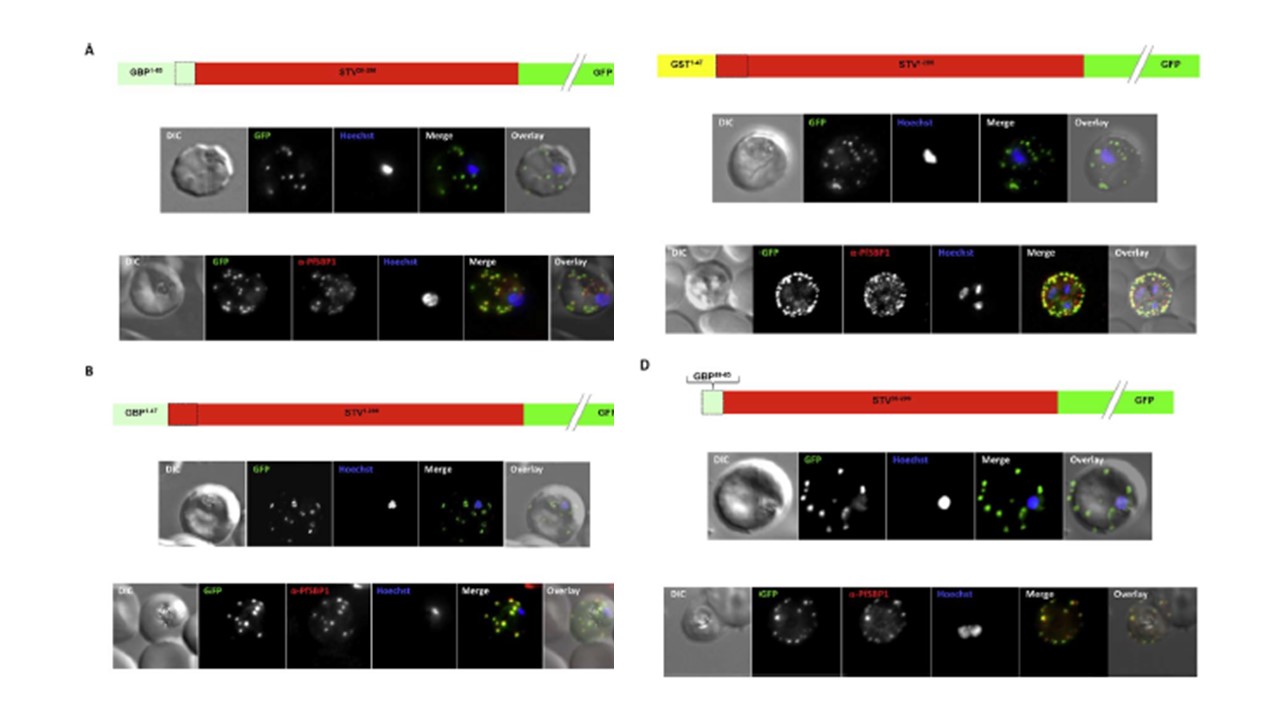
Effect of signal peptide type on export of a membrane protein. A schematic of each
construct is shown above the respective images. Upper panels show live cells, lower
panels fixed cells. DIC, differential interference contrast; GFP, green fluorescent protein; Hoechst, nuclear staining. In merge and overlay: green, GFP; blue, Hoechst; red, anti-SBP1. Having excluded an important role for N-terminal extensions in the export of soluble proteins, we wished to study whether the C- domain from GBP130 is alone capable of driving entry of a membrane protein to the secretory pathway. For this reason we expressed a construct composed of the C- domain of GBP130 (aa48-65) fused to STEVOR lacking the endogenous signal peptide. Once again we observed a high
level of export to the Maurer’s clefts, and co-localisation with PfSBP1
Meyer C, Barniol L, Hiss JA, Przyborski JM. The N-terminal extension of the P. falciparum GBP130 signal peptide is irrelevant for signal sequence function. Int J Med Microbiol. 2017 S1438-4221(17)30195-9.
Other associated proteins
| PFID | Formal Annotation |
|---|---|
| PF3D7_1016300 | glycophorin binding protein |