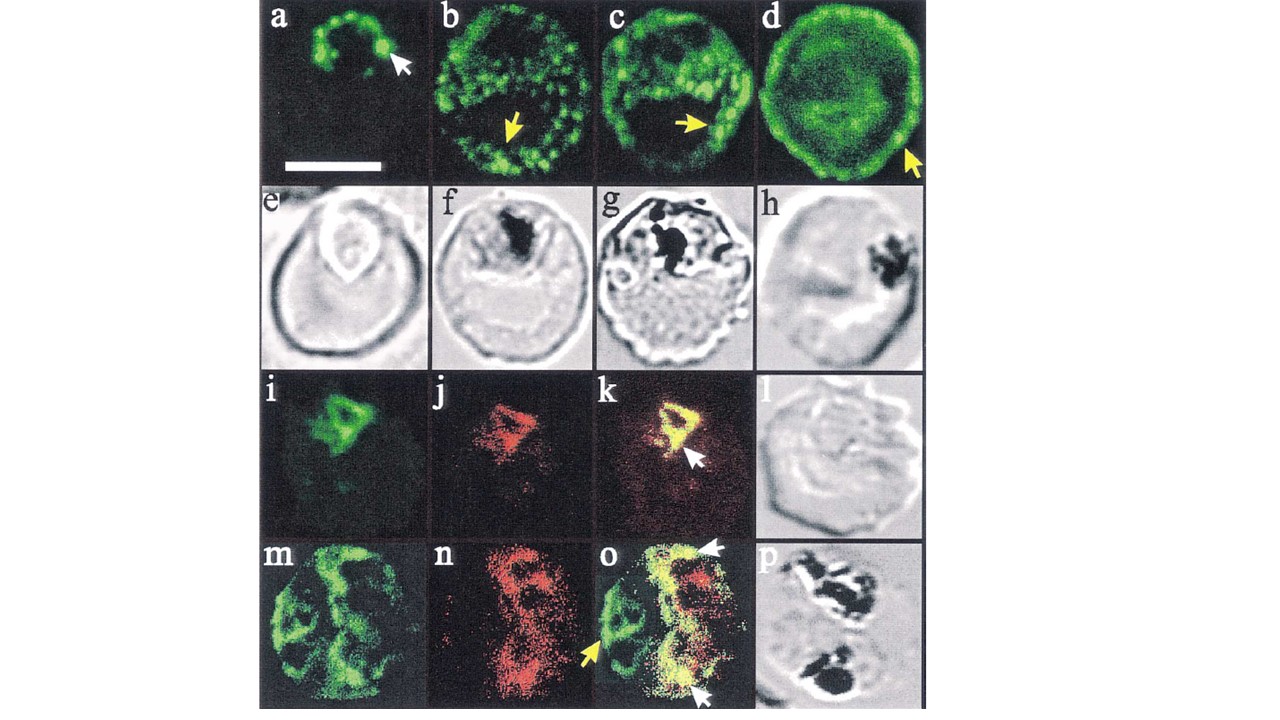
Confocal immunofluorescence of PfHRP2 and Pgh-1. Panels a–d: location of PfHRP2 in parasitised erythrocytes. Smears of cultured infected erythrocytes (3D7 strain) were probed with an anti-PfHRP2 monoclonal antibody (3C6) followed by a fluorescein-conjugated anti-mouse antiserum. A white arrow head (in panel a) marks ‘beads’ of PfHRP2 at the periphery of a late ring stage parasite. Some PfHRP2-containing structures within the erythrocyte cytosols of mid (panels b and c) and late stage (panel d) trophozoite-infected erythrocytes are marked with yellow arrow heads. Transmission images of panels a–d are shown in panels e–h. Panels i–p: dual labelling with anti-PfHRP2 and anti-Pgh-1. Smears of cultured infected erythrocytes (3D7 strain) were probed with a monoclonal anti-PfHRP2 antibody (3C6) followed by an fluorescein-conjugated anti-rabbit antiserum (green fluorescence) and an affinity purified rabbit antiserum recognising the food vacuole-located protein, Pgh-1, followed by rhodamine-conjugated anti-rabbit antiserum (red fluorescence). Some structures within the parasite that contain both PfHRP2 and Pgh-1 are marked with white arrow heads. A PfHRP2-containing structure in the erythrocyte cytosol is marked with a yellow arrow head. For each of the images, an optical slice was collected through the centre of the parasites by confocal microscopy. Dual images were collected simultaneously, and are displayed as isolated fluorescein fluorescence, isolated rhodamine fluorescence and overlayed fluorescence images of the same cell. The bar in panel (a) represents 5 mm.
Papalexis V, Siomos MA, Campanale N, Guo X, Kocak G, Foley M, Tilley L. Histidine-rich protein 2 of the malaria parasite, Plasmodium falciparum, is involved in detoxification of the by-products of haemoglobin degradation. Mol Biochem Parasitol. 2001 115(1):77-86.
Other associated proteins
| PFID | Formal Annotation |
|---|---|
| PF3D7_0523000 | multidrug resistance protein 1. MDR1, IP3 receptor |