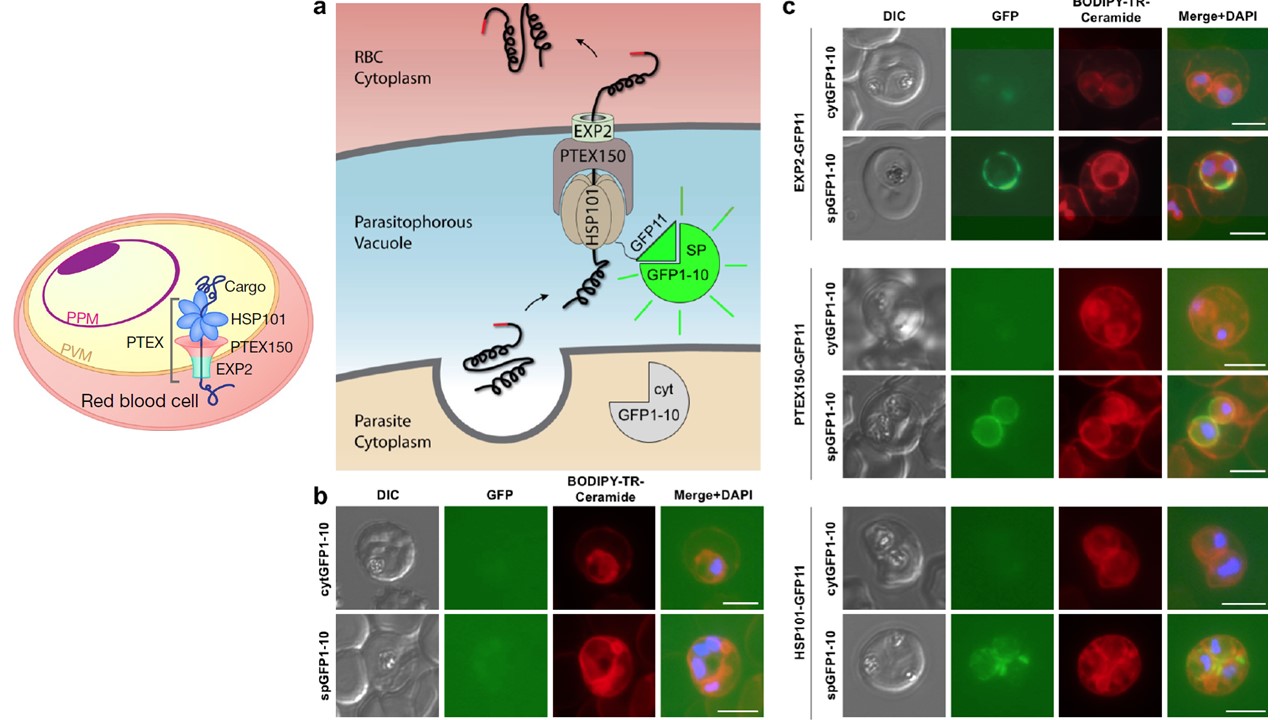
Split GFP assays argue against export-dedicated Sub-compartments and resolve EXP2 topology. a, Schematic showing split GFP experimental design. GFP1-10 was targeted to the parasite cytosol (cytGFP1-10) or PV lumen (by N-terminal fusion of a signal peptide, spGFP1-10). Endogenous GFP11 tags were made in the cytGFP1-10 or spGFP1-10 parasite backgrounds on the C-terminus of EXP2, PTEX150 or HSP101 (schematic shows HSP101-GFP11 as an example). b Live fluorescence imaging of parasite lines expressing cytGFP1-10 or spGFP1-10. No appreciable GFP signal is observed. Infected RBCs were labeled with BODIPY-TR-Ceramide to demarcate the PVM and other membranes. c, Live fluorescence imaging of cytGFP1-10 and spGFP1-10 parasite lines bearing endogenous GFP11 tags on EXP2, PTEX150 and HSP101. Vacuolar GFP signal is observed in the spGFP1-10 parasite background but not in the cytGFP1-10 parasite background. All GFP channel images were acquired with equal exposure time. Data are representative of two independent experiments. DIC, differential interference contrast. Scale bars, 5 μm.
Garten M, Nasamu AS, Niles JC, Zimmerberg J, Goldberg DE, Beck JR. EXP2 is a nutrient-permeable channel in the vacuolar membrane of Plasmodium and is essential for protein export via PTEX. Nat Microbiol. 2018 PMID: 30150733
Other associated proteins
| PFID | Formal Annotation |
|---|---|
| PF3D7_1116800 | heat shock protein 101 chaperone protein ClpB2 |
| PF3D7_1471100 | exported protein 2 |