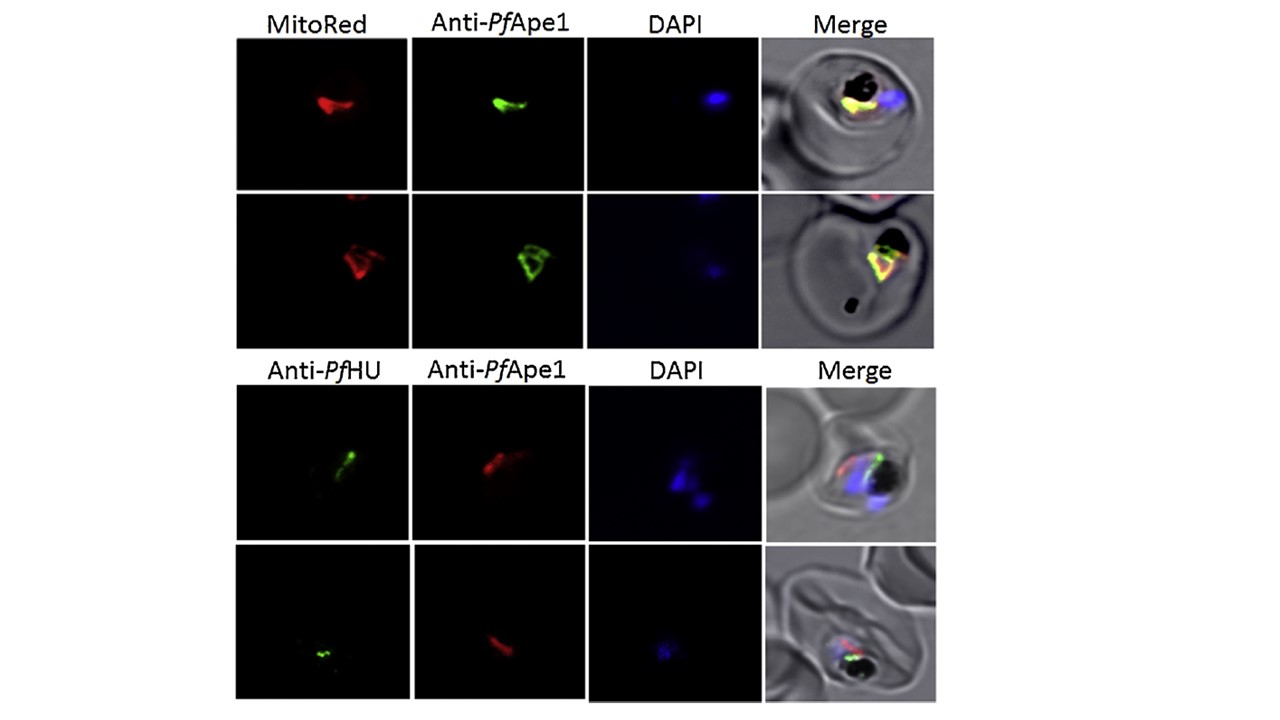
PfApe1 is targeted to the mitochondrion. co-localization
of PfApe1 signal with Mitotracker Red in P. falciparum-infected erythrocytes with the parasite in trophozoite stage (upper panel). PfApe1 signal did not overlap with apicoplast marker PfHU (lower panel). Parasite nuclei were stained with DAPI. Subcellular localization using purified Anti-PfApe1 Ab was determined by immunofluorescence assays. PfApe1 signals clearly co-localized with the mitochondrial marker dye Mitotracker Red; no overlap was observed with the apicoplast marker PfHU, hence confirming PfApe1 targeting to the parasite mitochondrion. The exclusive localization of PfApe1 in the mitochondrion suggests that either both the unprocessed and processed forms of the protein are associated with the organelle or that the processed form seen in parasite lysates is generated only upon cell lysis and is not produced endogenously. Thus the two AP endonucleases (PfApn1 and PfApe1) of the BER pathway target to the parasite mitochondrion, with no identifiable apicoplast or nuclear version.
Verma N, Shukla H, Tiwari A, Mishra S, Habib S. Plasmodium Ape1 is a multifunctional enzyme in mitochondrial base excision repair and is required for efficient transition from liver to blood stage infection. DNA Repair (Amst). 2021 101:103098.
Other associated proteins
| PFID | Formal Annotation |
|---|---|
| PF3D7_0305600 | AP endonuclease (DNA-[apurinic or apyrimidinic site] lyase), putative |